Abstract
Introduction
The increasing incidence of prostate cancer (PC) in China leads to a significant disease burden. Although three novel androgen inhibitors (darolutamide, apalutamide, and enzalutamide) have been approved for patients with high-risk non-metastatic castration-resistant prostate cancer (nmCRPC), the economic evaluation of these novel treatments in China remains unknown. In this study, we aimed to evaluate the cost–utility of darolutamide combined with androgen deprivation therapy (ADT), comparing with apalutamide + ADT and enzalutamide + ADT, in patients with high-risk nmCRPC from a healthcare system perspective in China.
Methods
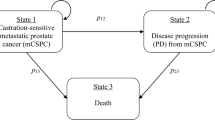
A partitioned survival model was developed to capture time spent by patients in three health states: nmCRPC, metastatic CRPC (mCRPC), and death. Clinical outcomes from the ARAMIS, PROSPER, and SPARTAN studies were obtained. In the absence of head-to-head studies, indirect treatment comparisons were conducted to capture the comparative effectiveness between darolutamide + ADT, apalutamide + ADT, and enzalutamide + ADT. The prices of apalutamide and enzalutamide were assumed to be the same as the initial launch price of darolutamide, since post-negotiation prices after national reimbursement drug list (NRDL) inclusion remain confidential. Other health resources costs, baseline characteristics, treatment patterns, and utility were collected through literature or clinical expert interviews. Selected sensitivity analyses were also performed.
Results
For a 20-year time horizon, darolutamide + ADT was associated with lower cost per quality-adjusted life years (QALYs) than apalutamide + ADT and enzalutamide + ADT (202,897 Chinese yuan (CNY)/QALY vs. 228,998 CNY/QALY and 221,409 CNY/QALY, respectively) (exchange rate, 1 USD = 6.7871 CNY). Darolutamide + ADT had better health outcomes and lower total costs compared to both apalutamide + ADT (+ 0.22 QALYs and − 72,818 CNY) and enzalutamide + ADT (+ 0.09 QALYs and − 67,451 CNY). Across the modelled sensitivity analyses (including hazard ratios and drug costs), darolutamide + ADT remained dominant or cost-effective.
Conclusions
This economic evaluation suggested that, in comparison with apalutamide + ADT and enzalutamide + ADT, darolutamide + ADT was a dominant or cost-effective treatment option for patients with high-risk nmCRPC in China.





Similar content being viewed by others
References
WHO. Estimated crude incidence/mortality rates in 2020 prostate, males, all ages. Global Cancer Observatory website. Retrieved 2021, from https://gco.iarc.fr/today/home.
Wang F, Wang C, Xia H, et al. Burden of prostate cancer in China, 1990–2019: findings from the 2019 global burden of disease study. Front Endocrinol (Lausanne). 2022;13: 853623. https://doi.org/10.3389/fendo.2022.853623.
Wong MC, Goggins WB, Wang HH, et al. Global incidence and mortality for prostate cancer: analysis of temporal patterns and trends in 36 countries. Eur Urol. 2016;70(5):862–74. https://doi.org/10.1016/j.eururo.2016.05.043.
Kimura T, Egawa S. Epidemiology of prostate cancer in Asian countries. Int J Urol. 2018;25(6):524–31. https://doi.org/10.1111/iju.13593.
Gu XY, Zhang RS, et al. Analysis on the trend of prostate cancer incidence and age change in cancer registration areas of China, 2000 to 2014. Chin J Prev Med. 2018;52(6):586–92. https://doi.org/10.3760/cma.j.issn.0253-9624.2018.06.006.
Scher HI, Solo K, Valant J, et al. Prevalence of prostate cancer clinical states and mortality in the United States: estimates using a dynamic progression model. PLoS One. 2015;10(10): e0139440. https://doi.org/10.1371/journal.pone.0139440.
Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(13):2918–25. https://doi.org/10.1200/JCO.2005.01.529.
Nørgaard M, Jensen A, Jacobsen JB, et al. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). J Urol. 2010;184(1):162–7. https://doi.org/10.1016/j.juro.2010.03.034.
McDougall JA, Bansal A, Goulart BH, et al. The clinical and economic impacts of skeletal-related events among medicare enrollees with prostate cancer metastatic to bone. Oncologist. 2016;21(3):320–6. https://doi.org/10.1634/theoncologist.2015-0327.
Howard LE, Moreira DM, De Hoedt A, et al. Thresholds for PSA doubling time in men with non-metastatic castration-resistant prostate cancer. BJU Int. 2017;120(5b):e80-e86. https://doi.org/10.1111/bju.13856.
Lokeshwar SD, Klaassen Z, Saad F. Treatment and trials in non-metastatic castration-resistant prostate cancer. Nat Rev Urol. 2021;18(7):433–42. https://doi.org/10.1038/s41585-021-00470-4.
Chinese Society of Clinical Oncology. Guidelines for the diagnosis and treatment of prostate cancer (2021 edition). 2021.
Liu G, Hu S, Wu J, et al. China guidelines for pharmacoeconomic evaluations (2020 edition). 2020.
National Bureau of Statistics. Statistic report on national economic and social development in 2021. Retrieved from http://www.stats.gov.cn/tjsj/zxfb/202202/t20220227_1827960.html.
Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928. https://doi.org/10.1136/bmj.d5928.
Signorovitch JE, Wu EQ, Yu AP, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics. 2010;28(10):935–45. https://doi.org/10.2165/11538370-000000000-00000.
National Bureau of Statistics. China population and employment statistics yearbook 2017.
Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382(23):2197–206. https://doi.org/10.1056/NEJMoa2003892.
Smith MR, Saad F, Chowdhury S, et al. Apalutamide and overall survival in prostate cancer. Eur Urol. 2021;79(1):150–8. https://doi.org/10.1016/j.eururo.2020.08.011.
Fizazi K, Shore N, Tammela TL, et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med. 2020;383(11):1040–9. https://doi.org/10.1056/NEJMoa2001342.
Li F, Zhu B, He Z, et al. Exploring the determinants that influence end-of-life hospital costs of the elderly in Shanghai, China. Biosci Trends. 2018;12(1):87–93. https://doi.org/10.5582/bst.2017.01244.
Paulden M. Why it’s time to abandon the ICER. Pharmacoeconomics. 2020;38(8):781–4. https://doi.org/10.1007/s40273-020-00915-5.
National Institute for Health and Care Excellence. TA387: abiraterone for treating metastatic hormone-relapsed prostate cancer before chemotherapy is indicated. 2016. Retrieved from https://www.nice.org.uk/guidance/ta387.
National Institute for Health and Care Excellence. TA377: enzalutamide for treating metastatic hormone-relapsed prostate cancer before chemotherapy is indicated. 2015. Retrieved from https://www.nice.org.uk/guidance/ta377.
McKay RR, Silver R, Bhak RH, et al. Treatment of metastatic castration resistant prostate cancer with radium-223: a retrospective study at a US tertiary oncology center. Prostate Cancer Prostatic Dis. 2021;24(1):210–9. https://doi.org/10.1038/s41391-020-00271-7.
Swinburn P, Lloyd A, Nathan P, et al. Elicitation of health state utilities in metastatic renal cell carcinoma. Curr Med Res Opin. 2010;26(5):1091–6. https://doi.org/10.1185/03007991003712258.
Doyle S, Lloyd A, Walker M. Health state utility scores in advanced non-small cell lung cancer. Lung Cancer. 2008;62(3):374–80. https://doi.org/10.1016/j.lungcan.2008.03.019.
Lloyd A, Nafees B, Narewska J, et al. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95(6):683–90. https://doi.org/10.1038/sj.bjc.6603326.
Nafees B, Stafford M, Gavriel S, et al. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84. https://doi.org/10.1186/1477-7525-6-84.
National Institute for Health and Care Excellence. TA660: darolutamide with androgen deprivation therapy for treating hormone-relapsed non-metastatic prostate cancer. Technology appraisal guidance. 2020. Retrieved from https://www.nice.org.uk/guidance/ta660.
National Comprehensive Cancer Network. Clinical practice guidelines in oncology prostate cancer version 1. 2021.
National Healthcare Security Administration. Interim measures for the administration of use of drugs covered by the basic medical insurance. 2020. Retrieved from http://www.nhsa.gov.cn/art/2020/7/31/art_104_6492.html.
Hird AE, Magee DE, Bhindi B, et al. A systematic review and network meta-analysis of novel androgen receptor inhibitors in non-metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2020;18(5):343–50. https://doi.org/10.1016/j.clgc.2020.02.005.
Kumar J, Jazayeri SB, Gautam S, et al. Comparative efficacy of apalutamide darolutamide and enzalutamide for treatment of non-metastatic castrate-resistant prostate cancer: a systematic review and network meta-analysis. Urol Oncol. 2020;38(11):826–34. https://doi.org/10.1016/j.urolonc.2020.03.022.
Mori K, Mostafaei H, Pradere B, et al. Apalutamide, enzalutamide, and darolutamide for non-metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis. Int J Clin Oncol. 2020;25(11):1892–900. https://doi.org/10.1007/s10147-020-01777-9.
Liu Z, Zhang T, Ma Z, et al. Systemic management for nonmetastatic castration-resistant prostate cancer: a systematic review and network meta-analysis. Am J Clin Oncol. 2020;43(4):288–97. https://doi.org/10.1097/coc.0000000000000660.
Cusano E, Lee-Ying RM, Boyne DJ, et al. Systemic therapy for nonmetastatic castrate-resistant prostate cancer (M0 CRPC): a systematic review and network meta-analysis (NMA). J Clin Oncol. 2020;38(6_suppl):113–113. https://doi.org/10.1200/JCO.2020.38.6_suppl.113.
Wenzel M, Nocera L, Collà Ruvolo C, et al. Overall survival and adverse events after treatment with darolutamide vs. apalutamide vs. enzalutamide for high-risk non-metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis. Prostate Cancer Prostatic Dis. 2021. https://doi.org/10.1038/s41391-021-00395-4.
Moilanen AM, Riikonen R, Oksala R, et al. Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci Rep. 2015;5:12007. https://doi.org/10.1038/srep12007.
Zurth C, Sandmann S, Trummel D, et al. Blood-brain barrier penetration of [14C]darolutamide compared with [14C]enzalutamide in rats using whole body autoradiography. J Clin Oncol. 2018;36(6_suppl):345–345. https://doi.org/10.1200/JCO.2018.36.6_suppl.345.
Riaz IB, Almutairi A, Lang DK, et al. Cost-effectiveness of novel antiandrogens (AAs) for treatment of nonmetastatic castrate-resistant prostate cancer (nmCRPC). J Clin Oncol. 2020;38(15_suppl):5583–5583. https://doi.org/10.1200/JCO.2020.38.15_suppl.5583.
Tsiatas M, van Oostrum I, Tritaki G, et al. PCN218 cost-effectiveness of apalutamide + ADT versus enzalutamide + ADT in non-metastatic castration resistant prostate cancer in Greece. Value Health. 2019;22:S478. https://doi.org/10.1016/j.jval.2019.09.414.
Acknowledgements
The authors gratefully acknowledge the participation of Yanjun Liu, Wenni Li, Weijia Lu (resigned employees of IQVIA) in assisting literature review, data collection and data analysis, and Yihan Liao from Real World Solutions, IQVIA China in assisting manuscript Proofreading.
Funding
Sponsorship for this study, and Rapid Service Fee were funded by Bayer Healthcare Company Ltd. Funding was not contingent upon publication of this article.
Author Contributions
Shanlian Hu contributed to main conceptual ideas and proof outline. Jian Ming and Yuxia Wu developed the model, performed the computations, interpreted results and wrote manuscript. Rong Han, Xing Xu and Reg Waldeck contributed to the data acquisition. All authors provided critical feedback and helped shape the manuscript, and approved the final version.
Disclosures
Jian Ming and Yuxia Wu are employees of Real World Solutions, IQVIA China. Rong Han, Xing Xu, Reg Waldeck are employees of the Bayer Healthcare Company Ltd. Shanlian Hu has nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. Authors had full permissions to access/use the data that was used in this economic evaluation.
Data Availability
All data generated or analyzed during this study are included in this published article or as supplementary information files.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ming, J., Wu, Y., Han, R. et al. Cost–Utility Analysis of Darolutamide Combined with Androgen Deprivation Therapy for Patients with High-Risk Non-Metastatic Castration-Resistant Prostate Cancer in China. Adv Ther 40, 1087–1103 (2023). https://doi.org/10.1007/s12325-022-02389-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02389-7