Abstract
Introduction
To assess the prevalence of fatigue and its association with disease activity and patient-reported outcomes among patients with ulcerative colitis (UC) or Crohn’s disease (CD).
Methods
Data from a cross-sectional survey conducted with gastroenterologists and their consulting adult patients with UC or CD were analyzed. Data were collected via gastroenterologist-completed patient record forms and patient-self completion forms. Patient demographics, clinical characteristics, disease activity and medication use were reported by the gastroenterologist, while current symptoms (fatigue, rectal urgency, abdominal pain, sleep disturbance), work productivity and the Short Inflammatory Bowel Disease Questionnaire (SIBDQ) were reported by the patient. Logistic regression models were used to identify measures associated with fatigue and expressed as odds ratio (OR) with 95% confidence interval. p < 0.05 was considered statistically significant.
Results
A total of 1057 patients with UC and 1228 patients with CD were included in this analysis. Fatigue was reported in 22.6% of UC and 26.0% of patients with CD. Higher proportion of patients with UC and fatigue had moderate/severe disease activity (p = 0.0001), had a higher Mayo score (5.0 vs. 4.0, p < 0.0001) and were unemployed (5.6% vs. 3.9%, p = 0.0149) compared to those without fatigue. In patients with CD reporting fatigue, a higher proportion were female (55.9% vs. 48.2%, p = 0.0193), were unemployed (5.8% vs. 4.9%, p = 0.0069), had moderate/severe disease (p < 0.0001) and had a higher mean Crohn’s Disease Activity Index score (145.0 vs. 96.2, p < 0.0001) than patients without fatigue. Patients with UC and fatigue had higher mean level of pain (p < 0.0001) and sleep disturbance (p < 0.0001), whereas patients with CD and fatigue had lower SIBDQ scores (p < 0.0001) and greater work impairment (p = 0.0015) than patients without fatigue. Abdominal pain (OR: 2.01, p = 0.001) and use of immunomodulators (OR: 1.69, p = 0.006) increased the odds of having fatigue in patients with UC. In patients with CD, abdominal pain (OR: 2.29, p < 0.001) and use of biologics or biosimilars (OR: 2.02, p = 0.003) increased the odds of having fatigue.
Conclusion
Fatigue is a common symptom among patients with UC or CD that is associated with higher levels of disease activity and decreased work productivity and is driven by various factors. A multidisciplinary approach may be needed to manage fatigue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Fatigue is twice as common in patients with inflammatory bowel disease (IBD) compared to those without and is known to be associated with disease severity |
There is limited evidence on prevalence of fatigue in patients with ulcerative colitis (UC) or Crohn’s disease (CD) and its impact on sleep quality, work productivity and quality of life (QoL). Therefore, we assessed the same in patients with UC or CD using data from the Adelphi IBD Disease Specific Programme™ |
What was learned from the study? |
Fatigue in patients with UC or CD affected sleep quality, work productivity and quality of life |
A greater proportion of patients with UC or CD reported fatigue with moderate or severe disease activity and a higher Mayo score and were unemployed compared to those without fatigue |
A multidisciplinary approach may be needed to manage fatigue in patients with UC or CD |
Introduction
Ulcerative colitis (UC) and Crohn’s disease (CD) are immune-mediated diseases of the gastrointestinal tract with complex etiology and are classified as inflammatory bowel disease (IBD) [1]. While rectal bleeding is a predominant clinical manifestation in UC, abdominal pain and diarrhea are the hallmark symptoms of CD [2, 3]. These symptoms have been extensively studied along with evaluation of objective inflammation such as endoscopy, biomarkers, etc. Thus, controlling these symptoms is a common goal in the treatment of the disease; however, many patients also suffer from debilitating symptoms such as fatigue, even when the disease is in clinical remission [4].
Fatigue is described as lack of energy or an overwhelming sense of tiredness with limitation of daily activities that is not relieved by rest [5]. Various factors such as iron deficiency, low levels of serum vitamin D and magnesium, sleep disturbance, alcohol misuse and emotional stress have been identified to contribute to fatigue in IBD [6]. Due to the underlying complexity of factors contributing to fatigue, treatments are often empirical, and resolution of symptoms is difficult to achieve. Although common, fatigue often goes under-reported and untreated and there is a need for systematic assessment of fatigue in patients with IBD [7].
Fatigue is twice as common in patients with IBD compared to those without IBD [8] and is more common in CD (48–62%) than in UC (42–47%) [9]. Being a dominant symptom [1], some degree of fatigue is reported in 86% of patients with CD having active disease [10] and ranges from 41 to 48% in IBD in remission. In addition, recent studies have shown that fatigue significantly impairs quality of life (QoL) and is associated with disease activity in patients with UC or CD [11, 12]. Furthermore, several studies have shown that fatigue is associated with disease severity [13, 14].
Despite evident patient impact, fatigue is a poorly understood manifestation and has received limited research attention in IBD. In particular, prescription of medications that may contribute to fatigue should be reviewed as seen in a longitudinal study wherein avoidance of steroids and cessation of immunomodulatory therapy in CD were predictors of improved physical and cognitive fatigue, respectively [15]. On the other hand, anti-TNF therapy (infliximab or adalimumab) has been shown to reduce the symptoms of fatigue [15, 16]. Additionally, although the impacts of disease activity, quality of sleep, QoL and psychological factors on fatigue have been independently studied in patients with UC or CD, none of the studies to date have evaluated all of these in the same sample of patients.
For these reasons, the goal of this study was to understand the prevalence of fatigue in a real-world sample of patients with UC or CD as well as its impact on sleep quality, work productivity and QoL of patients.
Methods
Study Design and Data Collection
This point-in-time study included data collected in 2017–2018 from patients with UC or CD from the Adelphi IBD Disease Specific Programme™ (DSP) [17]. The Adelphi DSP consists of data pertaining to treatment practice, symptom prevalence, patient demographics, clinical outcomes, medication utilization, healthcare utilization, productivity and health-related quality of life.
The survey was conducted among gastroenterologists and their consulting patients with UC or CD in France, Germany, Italy, Spain, the UK (UC: N = 787, CD: N = 900) and the USA (UC: N = 270, CD: N = 328). Data were collected via gastroenterologist-completed patient record forms (PRFs) and voluntarily completed patient-self completion forms (PSCs).
Using a check box, patients provided informed consent for use of their anonymized and aggregated data for research and publication in scientific journals. Data were collected in such a way that patients and physicians could not be identified directly; all data were aggregated and de-identified before receipt.
All questions used in the Inflammatory Bowel Disease-Disease Specific Programme were reviewed by an ethics review board; in the USA, all materials were reviewed and approved by the Western Institutional Review Board study exempt under the criteria 45 CFR §46.104(d)(2) (protocol number: AG8199), and in the EU materials were reviewed and approved by the Freiburger Ethik-Kommission International (protocol number: 017/1679). This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Study Population
To take part in the survey, physicians had to meet the following inclusion criteria: (1) must have been a gastroenterologist, (2) must be between 4 and 40 years since date of qualification as a physician, (3) must be actively involved in the management of patients with UC or CD and (4) must have treated at least seven patients with UC or CD in an average month. Participating patients aged ≥ 18 years, diagnosed with UC or CD, and those visiting a participating gastroenterologist were included. Only patients for whom both a PRF and a PSC had been completed were included in this analysis.
Outcomes and Statistical Analysis
Patient demographics, clinical characteristics, disease activity and medication use were reported by the gastroenterologist, while current symptoms during the time of consultation (fatigue, rectal urgency, abdominal pain, sleep disturbance), work productivity (using the Work Productivity and Activity Impairment [WPAI] questionnaire), EQ-5D and the Short Inflammatory Bowel Disease Questionnaire (SIBDQ) were reported by the patient. The WPAI generates four metrics: absenteeism, presenteeism, overall work impairment and activity impairment [18]. The SIBDQ consists of ten items covering four dimensions: bowel, systemic, emotional and social; it is scored on a seven-point scale with higher scores indicating a better HRQoL [19]. Numeric rating scales (of 0–10 [where 0 = none, 10 = extreme]) were used to assess level of pain and sleep disturbance. For the other symptoms, patients indicated whether they were present or absent from a list of symptoms.
Disease activity was measured using the Mayo score for patients with UC [20] and the Crohn’s Disease Activity Index (CDAI) for patients with CD [21]. The components for the Mayo scores were collected and derived from PRF data (i.e., those were not directly reported by the gastroenterologists) and the score for all patients was calculated using the PRF. Similarly, the CDAI components were collected and derived based on the data (i.e., those were not directly reported by the gastroenterologists) from the PRF and PSC.
All analyses were conducted for UC and CD separately. Patients were categorized as either having fatigue or not having fatigue based on their response on the PSC form, where they indicated whether they were currently suffering from tiredness/exhaustion/fatigue. Demographics, clinical characteristics, disease activity, symptoms, patient-reported outcomes and physician-reported anemia were compared between patients with and without fatigue using t-tests for continuous variables and Fisher’s exact or chi-squared test for categorical variables.
Logistic regression models were constructed to identify measures associated with fatigue and the following statistics were reported for each covariate as needed: the odds ratio (OR), coefficient estimate, standard error, chi-square test statistic, p-value and 95% confidence interval (CI). Standard errors in regressions were adjusted to allow for intragroup correlation within the reporting physicians. Substantive knowledge about the disease area was used to guide variable selection rather than using a statistical method of variable reduction [22]. All statistical tests performed were two-sided with a significance level of α = 0.05 and were performed on Stata software version 16 [23].
Results
Patient Demographics and Characteristics
A total of 1057 patients with UC and 1228 patients with CD were included in this analysis. Fatigue was reported in 22.6% (n = 239) of UC and 26.0% (n = 320) of patients with CD. Demographics of patients with and without fatigue for UC and CD are presented in Tables 1 and 2.
There was significant difference in employment status between patients with UC with fatigue than those without (p = 0.0149) and had a higher Charlson Comorbidity Index (0.2 vs. 0.1, p = 0.0432) (Table 1). A significantly higher proportion of patients with fatigue had moderate or severe disease activity (moderate: 47.3% vs. 45.8%, severe: 6.7% vs. 1.7%, p = 0.0001) and a higher Mayo score (5.0 vs. 4.0, p < 0.0001), including a significant difference in endoscopic findings (11.7% vs. 3.1% with severe disease, p < 0.0001) compared to those without fatigue. A higher proportion of patients with UC and fatigue were not in remission compared to those without fatigue (52.3% vs. 39.7%, p = 0.0013). Patients reporting fatigue had lower SIBDQ scores (45.0 vs. 54.0, p < 0.0001) and greater work impairment (34.4% vs. 26.9%, p = 0.0170) than those not reporting fatigue. A significantly higher proportion of patients reporting fatigue in UC received corticosteroids (79.5% vs. 70.3%, p = 0.0051) and biologics (46.9% vs. 37.2%, p = 0.0084) compared to those not reporting fatigue. Patients with UC and fatigue also had higher rates of rectal urgency (30.1% vs. 17.2%), abdominal pain (39.3% vs. 19.2%), loss of appetite (14.2% vs. 4.5%), flatulence (28.9% vs. 8.4%), anemia (15.1% vs. 2.3%), back pain (16.3% vs. 3.3%) and arthralgia (17.2% vs. 2.8%), all p < 0.0001. Patients reporting fatigue also had a higher mean level of pain (3.8 vs. 2.7, p < 0.0001), a higher mean level of sleep disturbance (3.7 vs. 2.2, p < 0.0001) and overall work impairment (29.7% vs. 27.9%, p = 0.0170) than patients not reporting fatigue. Among patients with UC, 55 were diagnosed with anemia and 1002 were diagnosed without anemia. Patients not diagnosed with anemia experienced fatigue less often (n = 799, 80%) when compared with patients diagnosed with anemia (n = 19, 35%, p < 0.0001, Table 3).
A significantly higher proportion of patients with CD and fatigue were female (55.9% vs. 48.2%, p = 0.0193), were unemployed (5.8% vs. 4.9%, p = 0.0069), had moderate or severe disease (47.8% vs. 35.9% and 9.1% vs. 4.0%, p < 0.0001) and had a higher mean CDAI score (145.0 vs. 96.2, p < 0.0001) compared to those without fatigue (Table 2). A higher proportion of patients with CD and fatigue were not in remission compared to those without fatigue (45.9% vs. 38.3%, p = 0.0493). Patients reporting fatigue also had lower SIBDQ scores (45.0 vs. 54.0, p < 0.0001) and greater work impairment (31.3% vs. 23.6%, p = 0.0015) than patients not reporting fatigue. A higher proportion of patients reporting fatigue received corticosteroids (79.7% vs. 69.9%, p = 0.0008) and biologics (59.4% vs. 41.9%, p < 0.0001) than patients not reporting fatigue. Patients with CD and fatigue had higher rates of rectal urgency (23.1% vs. 14.1%, p = 0.0003), abdominal pain (56.3% vs. 28.2%), abdominal cramps (51.2% vs. 25.6%), anorexia (24.4% vs. 6.9%), anemia (18.1% vs. 3.5%), back pain (18.1% vs. 3.5%), arthralgia (24.7% vs. 4.3%) and joint swelling (7.2% vs. 2.0%), all p < 0.0001. Patients reporting fatigue also had a higher mean level of pain (4.1 vs. 2.9) and a higher mean level of sleep disturbance (3.6 vs. 2.2) than patients not reporting fatigue (p < 0.0001). In the CD group, 72 patients were diagnosed with anemia and 1156 were not. Patients not diagnosed with anemia reported fatigue less often (n = 871, 75%) compared with those diagnosed with anemia (CD: n = 37, 51%, p < 0.0001, Table 3).
Factors Associated with Fatigue in Patients with UC or CD
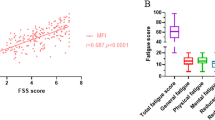
In patients with UC (n = 875), abdominal pain (OR: 2.01, 95% CI 1.31, 3.10, p = 0.001) and use of immunomodulators (OR: 1.70, 95% CI 1.16, 2.49, p = 0.006) significantly increased the odds of patients reporting fatigue. Patients with rectal bleeding had 0.09 times lower odds of reporting fatigue than those with ‘no blood seen’ (blood passed alone, OR: 0.09, 95% CI 0.01, 0.73, p = 0.024) compared with those not reporting fatigue. For every higher unit of the EQ-5D and SIBDQ score, patients were 0.12 times (OR: 0.12, 95% CI 0.03, 0.48, p = 0.003) and 0.56 times less likely to report fatigue (OR: 0.56, 95% CI 0.41, 0.77, p < 0.001), respectively (Fig. 1).
In patients with CD (n = 792), abdominal pain (OR: 2.29, 95% CI 1.45, 3.60, p < 0.001) and the use of biologics or biosimilars (OR: 2.02, 95% CI 1.27, 3.21, p = 0.003) significantly increased the odds of patients reporting fatigue. Patients with lower SIBDQ (OR: 0.54, 95% CI 0.39, 0.76, p < 0.001) and overall pain scores (OR: 0.82, 95% CI 0.71, 0.93, p = 0.003) were less likely to report fatigue (Fig. 2).
Discussion
This point-in-time survey of patients with UC or CD showed the association between fatigue and higher levels of disease activity, decreased work productivity and worse QoL. Approximately 26.0% of patients with CD and 22.6% of patients with UC reported fatigue. Disease activity was found to be associated with fatigue; patients with UC or CD reported were found to have higher mean Mayo and CDAI scores compared to those not reporting fatigue. A higher proportion of patients reporting fatigue had moderate or severe disease and were unemployed.
Graff et al. showed that fatigue can be present in IBD patients whose disease is in remission, which could be attributed to underlying psychological factors [13]. This is in accordance with the findings of this study wherein, despite still experiencing fatigue, 47.7% of UC and 54.1% of CD patients had achieved some form of remission. Fatigue may be a lasting systemic symptom of immune system challenge and/or may be a manifestation of persisting comorbid psychological difficulties, given the higher prevalence of mood disorders in IBD [24]. This study examined various factors for their probable association with fatigue. We found that several of them were associated independently with fatigue, highlighting the complex and multifactorial nature of fatigue in IBD. Rectal urgency, abdominal pain, a higher mean level of pain and sleep disturbance were associated with presence of fatigue in both UC and CD. However, while patients with fatigue were found to have significantly higher Mayo and CDAI scores compared to those without fatigue, disease activity was not found to be associated with the presence of fatigue. This finding is in line with previous research by Grimstad et al. [9].
Several explanations have been proposed for the association between sleep quality and fatigue in IBD. Poor sleep was found to be associated with increased bowel symptoms, which is a common concern among gastrointestinal disorders including UC and CD, while waking up due to pain can prevent patients from entering the restorative phase of deep sleep [25]. Additionally, a prolonged pain exposure (as seen in moderate and severe patients) can cause fatigue, as dealing with pain consumes energy [8]. Nevertheless, even if the mechanisms cannot be delineated clearly, the association of sleep quality with fatigue in IBD has implications for intervention because these are modifiable lifestyle factors.
Patients with IBD who reported fatigue were often noted to have poor QoL as reflected by a lower SIBDQ score. It is thought that fatigue leads to poor QoL and not vice versa [26]. IBD also has demonstrated an impact on work productivity and daily activities, thereby impairing QoL. Similarly, in our study, patients with UC or CD who reported fatigue also had lower SIBDQ scores and greater work impairment as measured by WPAI than patients not reporting fatigue.
Certain IBD medications have been thought to cause fatigue, and previous studies on sleep and fatigue considered the ongoing therapy to understand this relationship [27]. In this study, a higher proportion of patients with UC or CD reporting fatigue were currently receiving corticosteroids and biologics compared to patients not reporting fatigue. Although biologics can ameliorate fatigue symptoms and reduce disease activity, a significant proportion of IBD patients remain fatigued [28]. Patients receiving corticosteroids also experience more fatigue and overall lower QoL [29].
In IBD, anemia is a common side effect and has often been assumed to be a primary cause of fatigue [30]. It has been reported to be strongly associated with fatigue in IBD [8, 31], although findings were not consistent [10]. While we observed that patients not diagnosed with anemia experienced fatigue less often, we did not assess the association between presence of anemia and fatigue levels in our study. Interestingly, we also observed that 35% and 51% of patients with anemia in UC and CD groups, respectively, did not report fatigue. This could be due to adaptation of patients to chronic anemia or “asymptomatic” anemia, wherein patients adapt to low hemoglobin levels when anemia develops slowly [32]. Further investigations are therefore required to assess the relationship between anemia and fatigue.
Our study had some limitations. It included data from both the US and Europe, although it was not designed to systematically assess differences across regions. Our study design does not allow the causality evaluation of the relationships observed. Also, the disease activity was evaluated with standardized clinical indices only; such measurement may be inaccurate because there is limited correlation between symptom scores and endoscopic measures of inflammation.
Conclusion
Our study showed that patients with UC or CD reported fatigue and that fatigue was associated with poor QoL. Finally, our findings emphasized that fatigue in UC and CD is driven by various factors and that a multidisciplinary approach may be needed to manage fatigue. Further studies are required to determine whether medications can improve fatigue in this population and to shed new light on the etiology of fatigue in IBD.
References
Bączyk G, Kozłowska KA, Formanowicz D, Białas E, Karoń J, Krokowicz P. The relationship between the symptom of fatigue and the functioning of patients with inflammatory bowel diseases after surgery. Prz Gastroenterol. 2019;14(4):242–9.
Dignass A, Eliakim R, Magro F, Maaser C, Chowers Y, Geboes K, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6(10):965–90.
Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis. 2010;4(1):7–27.
Römkens TE, van Vugt-van Pinxteren MW, Nagengast FM, van Oijen MG, de Jong DJ. High prevalence of fatigue in inflammatory bowel disease: a case control study. J Crohns Colitis. 2011;5(4):332–7.
Barsevick AM, Cleeland CS, Manning DC, O’Mara AM, Reeve BB, Scott JA, et al. ASCPRO recommendations for the assessment of fatigue as an outcome in clinical trials. J Pain Symptom Manage. 2010;39(6):1086–99.
Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–106.
Czuber-Dochan W, Norton C, Bassett P, Berliner S, Bredin F, Darvell M, et al. Development and psychometric testing of inflammatory bowel disease fatigue (IBD-F) patient self-assessment scale. J Crohns Colitis. 2014;8(11):1398–406.
Jelsness-Jørgensen LP, Bernklev T, Henriksen M, Torp R, Moum BA. Chronic fatigue is more prevalent in patients with inflammatory bowel disease than in healthy controls. Inflamm Bowel Dis. 2011;17(7):1564–72.
Grimstad T, Norheim KB, Isaksen K, Leitao K, Hetta AK, Carlsen A, et al. Fatigue in newly diagnosed inflammatory bowel disease. J Crohns Colitis. 2015;9(9):725–30.
van Langenberg DR, Gibson PR. Systematic review: fatigue in inflammatory bowel disease. Aliment Pharmacol Ther. 2010;32(2):131–43.
IsHak WW, Pan D, Steiner AJ, Feldman E, Mann A, Mirocha J, et al. Patient-reported outcomes of quality of life, functioning, and GI/Psychiatric Symptom Severity in Patients with Inflammatory Bowel Disease (IBD). Inflamm Bowel Dis. 2017;23(5):798–803.
Cohen BL, Zoëga H, Shah SA, Leleiko N, Lidofsky S, Bright R, et al. Fatigue is highly associated with poor health-related quality of life, disability and depression in newly-diagnosed patients with inflammatory bowel disease, independent of disease activity. Aliment Pharmacol Ther. 2014;39(8):811–22.
Graff LA, Clara I, Walker JR, Lix L, Carr R, Miller N, et al. Changes in fatigue over 2 years are associated with activity of inflammatory bowel disease and psychological factors. Clin Gastroenterol Hepatol. 2013;11(9):1140–6.
Huppertz-Hauss G, Høivik ML, Jelsness-Jørgensen LP, Opheim R, Henriksen M, Høie O, et al. Fatigue in a population-based cohort of patients with inflammatory bowel disease 20 years after diagnosis: the IBSEN study. Scand J Gastroenterol. 2017;52(3):351–8.
Loftus EV, Feagan BG, Colombel JF, Rubin DT, Wu EQ, Yu AP, et al. Effects of adalimumab maintenance therapy on health-related quality of life of patients with Crohn’s disease: patient-reported outcomes of the CHARM trial. Am J Gastroenterol. 2008;103(12):3132–41.
Lichtenstein GR, Bala M, Han C, DeWoody K, Schaible T. Infliximab improves quality of life in patients with Crohn’s disease. Inflamm Bowel Dis. 2002;8(4):237–43.
Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: Disease-Specific Programmes—a means to understand. Curr Med Res Opin. 2008;24(11):3063–72.
Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–65.
Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol. 1996;91(8):1571–8.
Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317(26):1625–9.
Best WR, Becktel JM, Singleton JW. Rederived values of the eight coefficients of the Crohn’s Disease Activity Index (CDAI). Gastroenterology. 1979;77(4):843–6.
Heinze G, Wallisch C, Dunkler D. Variable selection—a review and recommendations for the practicing statistician. Biom J. 2018;60(3):431–49.
StataCorp. 2019. Stata Statistical Software: Release 16. College Station TSL.
Walker JR, Ediger JP, Graff LA, Greenfeld JM, Clara I, Lix L, et al. The Manitoba IBD cohort study: a population-based study of the prevalence of lifetime and 12-month anxiety and mood disorders. Am J Gastroenterol. 2008;103(8):1989–97.
Chrobak AA, Nowakowski J, Zwolińska-Wcisło M, Cibor D, Przybylska-Feluś M, Ochyra K, et al. Associations between chronotype, sleep disturbances and seasonality with fatigue and inflammatory bowel disease symptoms. Chronobiol Int. 2018;35(8):1142–52.
Hashash JG, Ramos-Rivers C, Youk A, Chiu WK, Duff K, Regueiro M, et al. Quality of sleep and coexistent psychopathology have significant impact on fatigue burden in patients with inflammatory bowel disease. J Clin Gastroenterol. 2018;52(5):423–30.
Marinelli C, Savarino EV, Marsilio I, Lorenzon G, Gavaruzzi T, D’Incà R, et al. Sleep disturbance in Inflammatory Bowel Disease: prevalence and risk factors—a cross-sectional study. Sci Rep. 2020;10(1):507.
Borren NZ, Tan W, Colizzo FP, Luther J, Garber JJ, Khalili H, et al. Longitudinal trajectory of fatigue with initiation of biologic therapy in inflammatory bowel diseases: a prospective cohort study. J Crohns Colitis. 2020;14(3):309–15.
Chavarría C, Casanova MJ, Chaparro M, Barreiro-de Acosta M, Ezquiaga E, Bujanda L, et al. Prevalence and factors associated with fatigue in patients with inflammatory bowel disease: a multicentre study. J Crohns Colitis. 2019;13(8):996–1002.
Goldenberg BA, Graff LA, Clara I, Zarychanski R, Walker JR, Carr R, et al. Is iron deficiency in the absence of anemia associated with fatigue in inflammatory bowel disease? Am J Gastroenterol. 2013;108(9):1392–7.
Romberg-Camps MJ, Bol Y, Dagnelie PC, Hesselink-van de Kruijs MA, Kester AD, Engels LG, et al. Fatigue and health-related quality of life in inflammatory bowel disease: results from a population-based study in the Netherlands: the IBD-South Limburg cohort. Inflamm Bowel Dis. 2010;16(12):2137–47.
Gasche C, Lomer MC, Cavill I, Weiss G. Iron, anaemia, and inflammatory bowel diseases. Gut. 2004;53(8):1190–7.
Acknowledgements
Funding
The DSP is a wholly owned Adelphi Real World multi-subscriber product. Eli Lilly and Company is one of multiple subscribers to the DSP. The journal’s Rapid Service and Open Access fees were funded by Eli Lilly and Company.
Medical Writing and Editorial Assistance
Era Seth, an employee of Eli Lilly Services India Pvt., Ltd., provided editorial support. Meena Ravuri, an employee of Eli Lilly Services India Pvt., Ltd., provided support in addressing peer review comments from the journal with contributions from the authors.
Author Contributions
TH, MR and ANN contributed to conception, design and interpretation of data. RL, RW and HK contributed to design and interpretation of the data. MS and RW were involved in the analysis of the data. TH and PB were involved in drafting the manuscript and critical revision. All the authors were involved in interpretation of the data and critical revision for the intellectual content.
Disclosures
RL, RW and HK: employees of Adelphi Real World. TH, ANN, MS and PB: employees of Eli Lilly and Company. MR: Advisory Boards and Consultant for Abbvie, Janssen, UCB, Takeda, Pfizer, Miraca Labs, Amgen, Celgene, Seres, Allergan, Genentech, Gilead, Salix, Prometheus, Lilly, TARGET Pharma Solutions, ALFASIGMA, S.p.A.
Compliance with Ethics Guidelines
Using a check box, patients provided informed consent for use of their anonymized and aggregated data for research and publication in scientific journals. Data were collected in such a way that patients and physicians could not be identified directly; all data were aggregated and de-identified before receipt. All questions used in the Inflammatory Bowel Disease-Disease Specific Programme were reviewed by an ethics review board; in the USA, all materials were reviewed and approved by the Western Institutional Review Board study exempt under the criteria 45 CFR §46.104(d)(2) (protocol number: AG8199), and in the EU materials were reviewed and approved by the Freiburger Ethik-Kommission International (protocol number: 017/1679). This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Data Availability
All data, i.e., methodology, materials, data and data analysis that support the findings of this survey, are the intellectual property of Adelphi Real World. All requests for access should be addressed directly to Rina Lukanova at rina.lukanova@adelphigroup.com.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Regueiro, M., Hunter, T., Lukanova, R. et al. Burden of Fatigue Among Patients with Ulcerative Colitis and Crohn’s Disease: Results from a Global Survey of Patients and Gastroenterologists. Adv Ther 40, 474–488 (2023). https://doi.org/10.1007/s12325-022-02364-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02364-2