Abstract
Introduction
Clinical data of esaxerenone in hypertensive patients with diabetic kidney disease (DKD) are lacking. We evaluated the efficacy and safety of esaxerenone in patients with DKD and an inadequate response to blood pressure (BP)-lowering treatment.
Methods
In this multicenter, open-label, prospective study, patients were divided into urinary albumin-to-creatinine ratio subcohorts (UACR < 30, 30 to < 300, and 300 to < 1000 mg/gCr). Esaxerenone was initiated at 1.25 mg/day and followed by incremental dose escalation based on BP and serum potassium level monitoring. The treatment period was 12 weeks. The primary endpoint was change in morning home systolic BP/diastolic BP (SBP/DBP) from baseline to end of treatment (EOT). Secondary endpoints included achievement rate of target BP, change in UACR from baseline, and safety.
Results
In total, 113 patients were enrolled. Morning home SBP/DBP significantly decreased from baseline to EOT in the total population (− 11.6/− 5.2 mmHg, both p < 0.001) and in all UACR subcohorts (all p < 0.001). The target BP achievement rate was 38.5%. Significant reductions in bedtime home and office BPs were also shown in the total population and all UACR subcohorts. UACR significantly improved from baseline to EOT in the total (− 50.9%, p < 0.001) and all UACR subcohorts (all p < 0.001). Incidence of serum potassium elevation as drug-related treatment emergent adverse events was 2.7%. The change from baseline in estimated glomerular filtration rate (eGFR) was − 4.8 mL/min/1.73 m2.
Conclusion
Esaxerenone demonstrated a BP-lowering effect and improved albuminuria. The effects were consistent regardless of the severity of albuminuria without clinically relevant serum potassium elevation and eGFR reduction.
Clinical Trial Registration
jRCTs06119002.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The progression of diabetic kidney disease (DKD) is associated with many risk factors, and the management of blood glucose levels alone is not sufficient to suppress DKD progression to more advanced stages; reducing glomerular hypertension through normalization of blood pressure (BP) is also particularly important to inhibit the progression. |
In many DKD cases, BP cannot be adequately controlled with BP-lowering treatment; esaxerenone, a next-generation mineralocorticoid receptor blocker, may be of benefit in these patients. |
This study investigated the efficacy and safety of esaxerenone in hypertensive patients with DKD who have an inadequate response to BP-lowering treatment with a renin–angiotensin system (RAS) inhibitor monotherapy or combined therapy with a calcium channel blocker (CCB). |
What was learned from the study? |
Esaxerenone reduced BP and improved albuminuria regardless of the severity of albuminuria and without clinically relevant serum potassium elevation or estimated glomerular filtration rate reduction. |
This is the first study to demonstrate that esaxerenone reduced not only office BP but also home BP in hypertensive patients with DKD. |
In hypertensive patients with DKD who do not respond well to RAS inhibitor treatment, esaxerenone may be a suitable alternative antihypertensive treatment option. |
Introduction
Diabetic kidney disease (DKD), also known as diabetic nephropathy, is caused by diabetes mellitus (DM). DKD is characterized by glomerular hyperfiltration in the early stage and gradually progresses to microalbuminuria, overt albuminuria, and nephrotic syndrome. In recent years, the number of patients with DKD manifesting with nephrotic syndrome has been decreasing owing to advances in diabetes treatment [1]. However, the number of patients with reduced estimated glomerular filtration rate (eGFR) without albuminuria is rising [2, 3]. Associated with eGFR decline in DKD, cerebrovascular and cardiovascular morbidity and mortality progressively increase; thus, therapeutic intervention from an early stage is crucial. Unfortunately, the number of patients with DKD is increasing worldwide, and the prevalence of DKD with micro- and macroalbuminuria among Japanese patients with type 2 DM has been reported to be more than 40% [4, 5].
The progression of DKD is associated with many risk factors such as hyperglycemia [6], hypertension [7], dyslipidemia [8], smoking [9], and age [10]; therefore, the management of blood glucose levels alone is not sufficient to suppress DKD progression to more advanced stages. In such cases, in addition to the management of blood glucose levels, inhibition of DKD progression by reducing glomerular hypertension through normalization of blood pressure (BP) is particularly important. Guidelines for antihypertensive treatment in patients with DKD recommend strict control of systemic BP (< 130/80 mmHg) and the use of renin–angiotensin system (RAS) inhibitors as first-line therapy to ameliorate glomerular hypertension and reduce proteinuria [11,12,13]. However, in clinical practice, there are many cases in which BP cannot be adequately controlled with RAS inhibitor monotherapy. In low renin hypertension, which is common in elderly patients, the antihypertensive effect of RAS inhibitors is attenuated [14, 15]. Additionally, long-term use of RAS inhibitors is associated with aldosterone breakthrough [16], in which aldosterone causes inflammation and interstitial fibrosis in kidney tissue. In fact, the incidence of aldosterone breakthrough has been reported to be 53% in patients using RAS inhibitors for more than 1 year [17]. Therefore, there is a need for the development of additional therapeutic options for hypertension management in patients with DKD.
Considering their antihypertensive and albuminuria-lowering effects, mineralocorticoid receptor blockers (MRBs) are expected to be a new option for the treatment of hypertension in patients with DKD. However, their clinical application has been limited owing to the sex hormone-related side effects of spironolactone [18] and the contraindication of eplerenone due to hyperkalemia in hypertensive patients with DM and proteinuria [19, 20]. Esaxerenone, which was approved as an antihypertensive drug in Japan in 2019 [21], is a next-generation MRB with high selectivity and with antihypertensive and urinary albumin-to-creatinine ratio (UACR)-lowering effects in hypertensive patients with DKD [22, 23]. In a previous study of hypertensive patients with moderate kidney dysfunction, the antihypertensive effects of esaxerenone on sitting systolic BP (SBP)/diastolic BP (DBP) were − 17.8 (95% confidence interval [CI] − 21.0, − 14.7)/− 8.1 mmHg (95% CI − 9.7, − 6.5), and esaxerenone significantly reduced the mean UACR from baseline to week 12 by 28.6% (95% CI − 40.7%, − 14.0%, p < 0.001) when administered as add-on therapy to RAS inhibitors [23]. In another study in hypertensive patients with type 2 DM and albuminuria on concomitant RAS inhibitors, esaxerenone had high antihypertensive and UACR-lowering effects (SBP/DBP, − 13.7/− 6.2 mmHg; UACR, − 32.4%) [22]. However, the number of patients with an eGFR between 30 and 60 mL/min/1.73 m2 in these two phase 3 trials was small, and clinical data on the efficacy and safety of esaxerenone in patients with moderate renal dysfunction are lacking. The EX-DKD study, one of the first studies of esaxerenone under the Clinical Trials Act, aimed to evaluate the efficacy and safety of esaxerenone in hypertensive patients with DKD who have an inadequate response to BP-lowering treatment with a RAS inhibitor or combination of a RAS inhibitor and calcium channel blocker (CCB).
Methods
Study Design
The EX-DKD study was a multicenter (22 sites), open-label, prospective study conducted in Japan from December 2019 to May 2022 (Fig. S1 in the supplementary material), and participating institutions and representative physicians are listed in Table S1 in the supplementary material.
Compliance with Ethics Guidelines
The study protocol was approved by the Okayama University Certified Review Board (CRB6180001) and prospectively registered with the Japan Registry of Clinical Trials (jRCTs061190027; https://jrct.niph.go.jp/en-latest-detail/jRCTs061190027). The study was conducted in accordance with the principles of the Declaration of Helsinki 1964, and its later amendments, and the Clinical Trials Act in Japan. All patients provided written informed consent before enrollment.
Patients
The inclusion criteria were as follows: patients aged 20 to < 85 years, with a diagnosis of type 2 DM (glycated hemoglobin < 9%), for whom home BP measurements could be obtained, who had received prior RAS inhibitor or RAS inhibitor plus CCB treatment before obtaining informed consent, had sitting office SBP of 130 to < 180 mmHg and/or DBP of 80 to < 110 mmHg at the start and end of the pre-study observation run-in period, had a UACR of < 1000 mg/gCr during the pre-study observational run-in period, and had a creatinine-based estimate of the glomerular filtration rate (eGFRcreat) of 30 to < 60 mL/min/1.73 m2. Major exclusion criteria were as follows: patients with secondary or malignant hypertension, type 1 DM, non-diabetic chronic kidney disease with increased or decreased doses of steroids or immunosuppressive drugs within 3 months before obtaining informed consent or with plans to increase or decrease doses of these drugs within the following 4 months, nephrotic syndrome, acute glomerulonephritis, complication or history of orthostatic hypotension, rapidly progressive glomerulonephritis, ankle-brachial index ≤ 0.9 in patients with symptoms caused by arteriosclerosis obliterans, cerebrocardiovascular disease, severe hepatic dysfunction, and serum potassium level > 5.0 mEq/L at the end of the pre-study observation run-in period. On the basis of baseline UACR, all patients were divided into two albuminuria subcohorts or three UACR subcohorts as follows: patients with a UACR of < 30 mg/gCr and 30 to < 1000 mg/gCr were assigned to the no albuminuria and albuminuria subcohorts, respectively, and patients with a UACR of < 30 mg/gCr, 30 to < 300 mg/gCr, and 300 to < 1000 mg/gCr were assigned to the A1, A2, and A3 subcohorts, respectively.
Treatments
After a 4-week run-in period, esaxerenone was initiated at a dose of 1.25 mg/day, and then could be gradually increased to 2.5 mg/day (week 4) and to 5 mg/day (week 8) on the basis of BP and serum potassium level monitoring. The entire treatment period was 12 weeks. Prior treatment with RAS inhibitor (or RAS inhibitor plus CCB) was continued at a constant dose throughout the study, and the dose and type of antihypertensive and antihyperglycemic drugs could not be changed. The following concomitant drugs were prohibited from 4 weeks before the treatment start to the end of the treatment (EOT) period or the time of discontinuation: antihypertensive and antianginal drugs (angiotensin receptor blockers [ARBs], angiotensin-converting enzyme inhibitors, CCBs, renin inhibitors, α-blockers, β-blockers, αβ-blockers, other sympatholytic agents, vasodilators), diuretics (thiazide, thiazide-like, loop, potassium-sparing diuretics), aldosterone antagonists, potassium preparations, non-steroidal anti-inflammatory drugs, and calcium polystyrene sulfonate.
Measurement of BP
The office BP and pulse rate were measured twice at each visit (at baseline; 2, 4, 8, and 12 weeks; end of the study; and discontinuation of the study), and the average of the two measurements was used. Office BP was measured at intervals of at least 3 h after meals and after at least 5 min of rest in a sitting position. When the clinical blood sampling was carried out, the BP and pulse rate were measured prior to the blood sampling. Home BP was self-measured twice (at morning and bedtime) using an upper arm cuff sphygmomanometer within the last 5 days of the patient’s visit, and the average of the two measurements was used. The sphygmomanometer owned by the patients was used throughout the study period. Morning home BP was measured after urination within 1 h after waking up and before breakfast, medication, and caffeine intake. Bedtime home BP was measured over 1 h after bathing, drinking, or caffeine intake before bedtime.
Measurement of Other Outcomes
At each visit (at baseline; 4, 8, and 12 weeks; and the time of discontinuation), the albumin and creatinine concentrations in the collected spot urine samples were measured by a central measurement laboratory (SRL, Inc., Tokyo, Japan), and the UACR was calculated using the following formula: UACR (mg/gCr) = urinary albumin (µg/mL)/urinary creatinine (mg/dL) × 100. Plasma aldosterone concentration (PAC) and plasma renin activity (PRA) were measured at baseline, 12 weeks, and at discontinuation in the central measurement laboratory (SRL, Inc.). PAC and PRA were measured after resting in the supine position for at least 30 min. Urinary concentrations of sodium, potassium, creatinine, protein/creatinine ratio, and biomarkers including liver-type fatty acid binding protein, N-acetyl-β-d-glucosaminidase, β2-microglobulin, angiotensinogen, and 8-hydroxydeoxyguanosine were measured at baseline, 12 weeks, and at discontinuation in the central measurement laboratory (SRL, Inc.). The eGFRcreat was calculated as follows: 194 × serum creatinine−1.094 × age−0.287, multiplied by 0.739 for female patients [24].
Efficacy Endpoints
The primary efficacy endpoint was the change in morning home SBP and DBP from baseline to EOT. Secondary efficacy endpoints were the change in bedtime home and office SBP and DBP from baseline to EOT, the time course change of home (morning, bedtime) and office SBP and DBP during the study, the achievement rate of target BP levels (SBP/DBP, home < 125/75 mmHg and office < 130/80 mmHg) at 12 weeks [11], the change and percentage change in UACR from baseline to EOT, and changes in serum and urinary biomarkers from baseline to 12 weeks.
Safety Endpoints
The safety endpoints included treatment-emergent adverse events (TEAEs), laboratory tests, vital signs, and 12-lead electrocardiography. The incidence of serum potassium level ≥ 5.5 and ≥ 6.0 mEq/L, time course changes in eGFR, and change from baseline in eGFR were also assessed as safety endpoints.
Statistical Analysis
We assumed that the change in sitting BP ± SD with esaxerenone would be − 8.7 ± 19.0 mmHg for SBP and − 4.9 ± 11.0 mmHg for DBP based on previous studies [25, 26]. Under this assumption, the statistical power was calculated as more than 90% when the analysis population was 55, and the significance level was set at a two-sided p value < 0.05. Considering five patients excluded from the analyses, the target sample size was set at 60 patients in each subcohort (no albuminuria and albuminuria subcohorts). Efficacy endpoints were analyzed in the full analysis set (FAS) that included all patients who provided informed consent, met the eligibility criteria, took at least one dose of esaxerenone, and had at least one efficacy measurement recorded. Point estimates and 95% CIs for the difference between baseline and EOT values were calculated and compared using the paired t test. EOT values were calculated by taking the average of measurements at the last two visits in the treatment period. The missing values at the EOT for sitting BP and UACR were imputed by the last observation carried forward method. Missing PAC, PRA, urinary biomarkers, and safety endpoints were not imputed. Safety endpoints were evaluated in the safety analysis set, defined as all enrolled patients who took at least one dose of esaxerenone, and were summarized using descriptive statistics. TEAEs were coded by System Organ Class and Preferred Term according to the Medical Dictionary for Regulatory Activities (MedDRA), version 24.1. Subgroup analysis was conducted according to baseline UACR (A1, A2, and A3). Multiplicity was not adjusted because of the small sample size, as this was an exploratory study. The significance level was set as 5% (two-sided). All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
A total of 113 patients were enrolled in the study (46, 43, and 24 in the A1, A2, and A3 subcohorts, respectively), of whom 109 were included in the FAS and 112 were included in the safety analysis set. A total of 95 patients completed the study. In total, four patients withdrew from the study because enrollment was suspended owing to the declaration of a state of emergency caused by the COVID-19 pandemic. One patient was excluded from the safety analysis set for not receiving treatment with esaxerenone, and three patients were excluded from the FAS for not meeting the inclusion criteria after taking esaxerenone. Baseline characteristics of the study population are shown in Table 1. In the total population, the mean age was 72.6 years; morning, bedtime, and office BP was 135.6/75.9 mmHg, 129.3/71.0 mmHg, and 144.7/76.1 mmHg, respectively; mean UACR was 184.0 mg/gCr; eGFRcreat was 49.4 mL/min/1.73 m2; serum potassium was 4.3 mEq/L; and glycated hemoglobin was 6.9%. The BP tended to be higher in the A3 subcohort compared with the other two UACR subcohorts, and was similar in the A1 and A2 subcohorts. In the A1, A2, and A3 subcohorts, the mean UACR was 13.8, 127.1, and 618.6 mg/gCr; mean eGFRcreat was 49.9, 49.8, and 47.8 mL/min/1.73 m2; and mean serum potassium was 4.3, 4.3, and 4.2 mEq/L, respectively. There were no notable differences in eGFRcreat and serum potassium levels between the three UACR subcohorts. The proportion of patients using RAS inhibitor plus CCB was 67.0% in the total population and 55.6%, 73.2%, and 78.3% in the A1, A2, and A3 subcohorts, respectively. The dose of esaxerenone was 1.25, 2.5, and 5 mg/day in 64 (58.7%), 36 (33.0%), and 9 (8.3%) patients, based on the prespecified dosage titration method at EOT (Table S2 in the supplementary material). According to UACR subcohorts, the proportion of patients with dose escalation to 2.5 or 5 mg/day was 20.0% in the A1 subcohort, 46.3% in the A2 subcohort, and 60.9% in the A3 subcohort.
BP-Lowering Effects of Esaxerenone
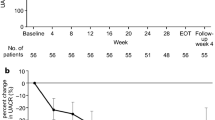
In the FAS, there were significant reductions in morning home SBP/DBP from the baseline to EOT (− 11.6 ± 9.2/− 5.2 ± 5.4 mmHg, both p < 0.001) (Fig. 1a). A significant reduction in SBP/DBP was also observed in the three UACR subcohorts (A1, − 12.5/− 6.2 mmHg; A2, − 11.5/− 4.2 mmHg; A3, − 9.9/− 5.0 mmHg; all p < 0.001) (Fig. 1b). Similar to the morning home BP, significant reductions were shown in bedtime home and office SBP/DBP (all p < 0.001, except for office DBP in the A1 subcohort and SBP/DBP in the A2 subcohort, all p < 0.01) (Fig. 1c, d). Morning home SBP/DBP decreased incrementally throughout the study period in the total population and UACR subcohorts (Fig. 2, Fig. S2 in the supplementary material). Similar reductions were also obtained in bedtime home and office BP (Table S3, Figs. S3 and S4 in the supplementary material). The achievement rate of target morning home SBP level at week 12 was 38.5% in the total population and 53.3%, 31.7%, and 21.7% in the A1, A2, and A3 subcohorts, respectively (Table S4 in the supplementary material).
Change from baseline in morning home BP levels at EOT in the total population (a) and urinary albumin-to-creatinine ratio subcohorts (b), and change from baseline in bedtime home BP level (c) and office BP level (d) (full analysis set). Patients with a urinary albumin-to-creatinine ratio of < 30 mg/gCr, 30 to < 300 mg/gCr, and 300 to < 1000 mg/gCr were assigned to the A1, A2, and A3 subcohorts, respectively. Data are mean [95% confidence interval]. **p < 0.001. BP blood pressure, DBP diastolic blood pressure, EOT end of treatment, SBP systolic blood pressure
Effects of Esaxerenone on UACR
The UACR decreased from baseline to EOT in the total population (the mean change from baseline to EOT was − 97.5 mg/gCr in the total population and − 4.2, − 59.8, and − 327.8 mg/gCr in the A1, A2, and A3 subcohorts, respectively (Table S5 in the supplementary material). The percentage change in UACR from baseline to EOT was − 50.9% (p < 0.001) (Fig. 3a, Table S5), and also significantly improved in all UACR subcohorts (− 34.2%, − 56.9%, and − 63.6% in the A1, A2, and A3 subcohorts, respectively; all p < 0.001) (Fig. 3b, Table S5).
Geometric percentage change in UACR from baseline to EOT in the total population (a) and UACR subcohorts (b) (full analysis set). Patients with a UACR of < 30 mg/gCr, 30 to < 300 mg/gCr and 300 to < 1000 mg/gCr were assigned to the A1, A2, and A3 subcohorts, respectively. Error bars indicate 95% confidence intervals. **p < 0.001. EOT end of treatment, UACR urinary albumin-to-creatinine ratio
Effects of Esaxerenone on Biomarkers
Biomarker data at baseline and week 12 are shown in Table S6 in the supplementary material. PAC and PRA numerically increased from baseline to week 12 in the total population (PAC, mean ± SD change, 24.6 ± 37.4 pg/mL; PRA, mean ± SD change, 4.0 ± 10.7 ng/mL/h), and also increased in all UACR subcohorts.
Safety
The proportion of patients with at least one TEAE was 23.2% (Table 2). Drug-related TEAEs were reported in eight (7.1%) patients, of whom four (3.6%) discontinued the study treatment owing to drug-related TEAEs. A serious TEAE of a fracture in the upper extremity was reported in one (0.9%) patient as an accidental fall. The most frequent TEAE was dizziness (2.7%). Drug-related TEAEs related to serum potassium elevation were hyperkalemia in two (1.8%) patients and blood potassium increased in one (0.9%) patient; the two patients with hyperkalemia discontinued the study. No cardiovascular-related adverse events; treatment-related trends regarding vital signs, 12-lead electrocardiogram, laboratory tests, or physical examinations; or deaths were reported during the treatment period. The eGFRcreat gradually decreased during this study and the mean change from baseline to week 12 was − 4.8 ± 5.4 mL/min/1.73 m2 (Fig. 4a; Fig. S5a in the supplementary material). Mean changes in eGFR from baseline to week 12 were similar between the UACR subcohorts (Fig. 4b; Fig. S5b in the supplementary material). Serum potassium levels increased over the first 2 weeks after starting esaxerenone treatment and remained stable during the treatment period (Fig. 4c, d; Fig. S5c, d in the supplementary material). Three (2.7%) patients had serum potassium levels ≥ 5.5 mEq/L during the study period, including two (4.3%) patients in the A1 subcohort and one (2.4%) patient in the A2 subcohort. No patients had serum potassium ≥ 6.0 mEq/L (Table S7 in the supplementary material).
Time course changes in eGFRcreat (a, b) and serum potassium levels (c, d) during the study period in the total population (a, c) and urinary albumin-to-creatinine ratio subcohorts (b, d) (safety analysis set). Patients with a urinary albumin-to-creatinine ratio of < 30 mg/gCr, 30 to < 300 mg/gCr, and 300 to < 1000 mg/gCr were assigned to the A1, A2, and A3 subcohorts, respectively. Data are mean ± standard deviation. eGFRcreat creatinine-based estimate of the glomerular filtration rate
Discussion
The EX-DKD study is one of the first clinical studies to evaluate the efficacy and safety of the non-steroidal MRB esaxerenone in patients with hypertension and DKD who have an inadequate response to BP-lowering treatment with a RAS inhibitor or combination of a RAS inhibitor and CCB. In previous phase 3 studies of esaxerenone, the study population of patients with an eGFR of 30 to 60 mL/min/1.73 m2 has been small [22, 23]. Thus, this study reinforces the evidence for esaxerenone in patients with moderate renal dysfunction.
Esaxerenone significantly reduced morning home SBP/DBP, as well as bedtime home and office SBP/DBP from baseline to EOT in the total population and in the three UACR subcohorts, indicating that esaxerenone exhibits an antihypertensive effect despite the severity of albuminuria. Office BP measurements enable us to compare the results of the present study with studies of other MRBs including previous phase 3 studies of esaxerenone. In the phase 3 studies of esaxerenone in hypertensive patients with moderate renal dysfunction [23] and in hypertensive patients with type 2 DM and microalbuminuria [22], conducted with the same dose-titration protocol as the EX-DKD study in the presence of a RAS inhibitor, the changes in office BP were − 17.8/− 8.1 mmHg [23] and − 13.7/− 6.2 mmHg [22], respectively. Study populations of these two studies are comparable to the A1 and A2 subcohorts in the EX-DKD study, and the reduction in office BP in the current study in these two subcohorts (A1, − 14.4/− 4.1 mmHg; A2, − 9.1/− 6.0 mmHg) was lower compared with the results of the previous phase 3 studies. This may be because the baseline BP value of the EX-DKD study was 144.7/76.1 mmHg, which is approximately 13–16 mmHg lower than the respective baseline office BP in these phase 3 trials (159.4/91.8 mmHg [23] and 158.7/89.0 mmHg [22]). In fact, in another phase 3 placebo-controlled study (ESAX-DN) in hypertensive patients with type 2 DM and microalbuminuria [27] who were receiving treatment with a RAS inhibitor, in which the baseline office BP value was 140.2/83.1 mmHg, the change in office BP from baseline to 12 weeks was − 8.9/− 3.7 mmHg, which was similar to that of the A2 population in the present EX-DKD study. Although this study lacked a placebo control group, the obtained antihypertensive effect was consistent with the placebo-controlled ESAX-DN study associated with similar increases in PRA and PAC, indicators of MR activity inhibition [28]. Thus, a placebo antihypertensive effect was not the main contributor for the reduction in BP. Previous studies evaluating the antihypertensive effect of MRBs in patients with moderate kidney dysfunction are lacking; therefore, available data are limited to patients with type 2 DM and albuminuria, and the direct comparison of our findings with studies of other MRBs is difficult. However, as far as we can consider from the current findings and available data, the antihypertensive effect of esaxerenone in these populations appears to be similar to that of spironolactone [29,30,31], and superior to those of eplerenone [32] and finerenone (FIDELIO-DKD trial) [33]. For example, in the FIDELIO-DKD trial, finerenone showed a modest effect on SBP in the presence of a RAS inhibitor at month 1 and 12 after starting treatment, and the mean changes in SBP from baseline to month 1 and 12 were − 3.0 and − 2.1 mmHg, respectively [33]. Furthermore, a recent meta-analysis of randomized controlled trials in patients with chronic kidney disease and type 2 DM reported that esaxerenone has a greater SBP-reducing effect than finerenone [34].
In the present study, the doses of esaxerenone at EOT remained at 1.25 mg in 58.7% of patients, especially in the A1 subcohort (without albuminuria), where 77.8% of patients received a final dose of 1.25 mg. This was attributed to the fact that morning home, bedtime home, and office DBP at baseline were nearly equal to or below the target BP in the Japanese Society of Hypertension guideline [11], and reached the target even at 1.25 mg; thus, no dose increase was necessary. Another possible reason for not increasing the dose is that the average age of the subjects was relatively high in the total population, 72.6 years (88.1% over 65 years), which may have caused physicians to be cautious about excessive hypotension. Thus, although more than half of the patients remained at a dose of 1.25 mg, esaxerenone exhibited a robust antihypertensive effect even at small doses in patients with DKD and inadequate response to BP-lowering treatment, especially in patients without albuminuria.
In patients with chronic kidney disease, albuminuria is reported to be a strong prognostic factor for the progression of kidney disease [35,36,37]. In patients with DKD, suppression of the UACR is associated with a reduction of the occurrence of end-stage renal disease. In the current study, even in the presence of a RAS inhibitor, esaxerenone significantly improved the UACR from baseline to EOT both in the total population (− 50.9%, p < 0.001) and in all the UACR subcohorts (− 34.2%, − 56.9%, and − 63.6% in the A1, A2, and A3 subcohorts, respectively; all p < 0.001). This reduction of UACR in the albuminuria subcohorts (A2 and A3 subcohorts) was likely superior to the results of previous phase 3 studies of esaxerenone in patients with DM and albuminuria (J306 and the ESAX-DN studies for microalbuminuria: − 32.4% [22] and − 43.8% [27] at 12 weeks; and J309 study for macroalbuminuria: − 34.4% at 12 weeks) [38] and also likely superior to that of finerenone (FIDELIO-DKD trial: 31% reduction in UACR from baseline to month 4 [32]; FIGARO-DKD trial: the reduction in UACR from baseline to month 4 was 32% greater with finerenone vs placebo [39]). In the previous phase 3 ESAX-DN study, it took 24 weeks for the albuminuria to reach a steady state after treatment with esaxerenone [27], and the UACR reduction observed at 12 weeks in this study is expected to be further reduced by continuous treatment with esaxerenone. The difference between these studies and the EX-DKD study is that the latter was limited to patients with albuminuria and moderate renal dysfunction. Considering that the present study included relatively elderly patients (mean age 72.6 years), a stratified analysis with a detailed patient background is needed.
Hyperkalemia is one of the known side effects of MRBs [40]. In the present study, hyperkalemia and blood potassium increased were also observed as drug-related TEAEs in two and one patients, respectively, and two patients with hyperkalemia discontinued the study. Risk factors for serum potassium elevation include moderate renal dysfunction, elderly patients, diabetes complications, high serum potassium levels, and concomitant treatment with RAS inhibitors [40]. Therefore, the patients enrolled in the present study, who were relatively elderly and had moderate renal dysfunction with RAS inhibitor treatment, had a predisposition to serum potassium elevation. However, the incidence of serum potassium level ≥ 5.5 and ≥ 6.0 mEq/L were 2.7% and 0%, respectively, which were similar to or lower than those in the previous phase 3 studies of esaxerenone in hypertensive patients with moderate renal dysfunction and type 2 DM with microalbuminuria (≥ 5.5 mEq/L, 3.9% [22] and 12.1% [23]; ≥ 6.0 mEq/L, both 0% [22, 23]). Two main reasons for the somewhat low incidence of serum potassium elevation are as follows: first, more than half of the patients received a final dose of 1.25 mg; second, the patients who received more than 2.5 mg underwent a titrated dosing regimen. With titrated dosing, serum potassium rises at the beginning of dosing and remains constant despite subsequent dose increases [40]. In this study, the same time-course transition of serum potassium levels was observed. Stratified analysis with the albuminuria subcohorts showed that the changes in serum potassium levels in each A1, A2, and A3 subcohort were similar to those in the total population, resulting in no differences in trends by albuminuria severity. These findings indicate that serum potassium elevation due to the addition of esaxerenone to therapy with an ARB or ARB plus CCB can be considered clinically manageable by adjusting the dosage from 1.25 to 5 mg/day in patients with DKD and moderate renal disfunction. The eGFR decreased to − 2.9 mL/min/1.73 m2 during the first 2 weeks of treatment, followed by a gradual decrease to − 4.8 mL/min/1.73 m2 at 12 weeks. The time-course transition of eGFR was not different in the A1, A2, and A3 subcohorts, and was in the same degree as previously reported studies of esaxerenone. It is known that there was a small and temporary reduction in eGFR after starting treatment with an MRB or RAS inhibitor [41, 42]. This decrease in eGFR is associated with normalization of glomerular hyperfiltration due to hemodynamic changes corresponding to BP changes, and the decrease in eGFR observed in this study was not considered a clinical safety concern. Regarding other safety factors, the incidence of TEAEs was similar to that reported in previous studies [22, 23], and no new safety concerns were raised. The findings of the present study suggest that esaxerenone can be used safely regardless of the UACR category, although careful attention must be paid to elevated serum potassium levels and decreased eGFR.
Limitations
Some limitations should be considered when interpreting the findings of this study. The enrolled patient population was relatively small, which may lead to a risk of low power of detection. This study did not incorporate a placebo control group, so it is possible that the reduction in BP observed was partly attributable to the placebo effect, or factors beyond the drug treatment, including regression to the mean. This limitation may also influence the observed UACR-lowering effect of esaxerenone. However, a previous placebo-controlled esaxerenone clinical trial has confirmed the significant antihypertensive and UACR-lowering actions of the drug [27, 43]. The duration of the study, with a 12-week evaluation period, may not be sufficient to determine the safety of esaxerenone in terms of its effect on serum potassium and eGFR. RAS inhibitors and sodium-glucose co-transporter 2 inhibitors cause a transient decrease in eGFR in the early phase of their administration and a subsequent slower decrease in eGFR. Although this phenomenon is known to have no clinical relevance [44], long-term evidence is lacking for esaxerenone. Additionally, patients with early DKD and a slow rate of eGFR decline were not enrolled in the study, and the long-term safety in patients with early DKD remains elusive. Similar long-term studies would be needed for esaxerenone. Few clinical studies have measured the efficacy of MRB on home BP in patients with DKD-associated hypertension, and comparisons with previously published studies can only be made using office BP. Finally, this study was conducted in Japan, and the outcomes may vary in other geographic locations and ethnic groups.
Conclusion
In Japanese hypertensive patients with DKD and moderate renal impairment who were treated with a RAS inhibitor or RAS inhibitor plus CCB, esaxerenone demonstrated a BP-lowering effect and improvement in albuminuria. The beneficial effects were consistent regardless of the severity of albuminuria. In addition, esaxerenone can be used safely in these patients without clinically relevant serum potassium elevation and eGFR reduction. Esaxerenone may be a suitable antihypertensive treatment option for hypertensive patients with DKD with inadequate response to BP-lowering treatment with a RAS inhibitor.
References
Kume S, Araki SI, Ugi S, et al. Secular changes in clinical manifestations of kidney disease among Japanese adults with type 2 diabetes from 1996 to 2014. J Diabetes Investig. 2019;10:1032–40.
Yokoyama H, Sone H, Oishi M, et al. Prevalence of albuminuria and renal insufficiency and associated clinical factors in type 2 diabetes: the Japan Diabetes Clinical Data Management study (JDDM15). Nephrol Dial Transplant. 2009;24:1212–9.
Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA. 2016;316:602–10.
Ohta M, Babazono T, Uchigata Y, Iwamoto Y. Comparison of the prevalence of chronic kidney disease in Japanese patients with type 1 and type 2 diabetes. Diabet Med. 2010;27:1017–23.
Gheith O, Farouk N, Nampoory N, Halim MA, Al-Otaibi T. Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol. 2015;5:49–56.
Hsu CC, Chang HY, Huang MC, et al. HbA1c variability is associated with microalbuminuria development in type 2 diabetes: a 7-year prospective cohort study. Diabetologia. 2012;55:3163–72.
Yeh CH, Yu HC, Huang TY, et al. The risk of diabetic renal function impairment in the first decade after diagnosed of diabetes mellitus is correlated with high variability of visit-to-visit systolic and diastolic blood pressure: a case control study. BMC Nephrol. 2017;18:99.
Chang YH, Chang DM, Lin KC, Hsieh CH, Lee YJ. High-density lipoprotein cholesterol and the risk of nephropathy in type 2 diabetic patients. Nutr Metab Cardiovasc Dis. 2013;23:751–7.
Rossing K, Christensen PK, Hovind P, Tarnow L, Rossing P, Parving HH. Progression of nephropathy in type 2 diabetic patients. Kidney Int. 2004;66:1596–605.
Iwase M, Ide H, Ohkuma T, et al. Incidence of end-stage renal disease and risk factors for progression of renal dysfunction in Japanese patients with type 2 diabetes: the Fukuoka Diabetes Registry. Clin Exp Nephrol. 2022;26:122–31.
Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481.
Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–248. (Erratum in J Am Coll Cardiol. 2018;71:2275–9).
Williams B, Mancia G, Spiering W, et al. ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041. (Erratum in J Hypertens. 2019;37:226).
Myron HW, William BW, Luis-Miguel R, et al. Effects of eplerenone versus losartan in patients with low-renin hypertension. Am Heart J. 2005;150:426–33.
Jöel B, Bernard IL, Jean-Baptiste M. Changes in the renin-angiotensin-aldosterone axis in later life. Drugs Aging. 1994;5:391–400.
Donderski R, Bednarski R, Manitius J. Controversy over renin–angiotensin–aldosterone system (RAAS) inhibitors treatment in nephrology and cardiovascular diseases. Arterial Hypertens. 2020;24:45–55.
Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol. 2007;3:486–92.
Colussi G, Catena C, Sechi LA. Spironolactone, eplerenone and the new aldosterone blockers in endocrine and primary hypertension. J Hypertens. 2013;31:3–15.
INSPRA (eplerenone) tablets. Highlights of prescribing information. 2008. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021437s006lbl.pdf.
Selara (eplerenone) Japanese package insert. https://pins.japic.or.jp/pdf/newPINS/00053675.pdf.
Duggan S. Esaxerenone: first global approval. Drugs. 2019;79:477–81.
Itoh H, Ito S, Rakugi H, Okuda Y, Nishioka S. Efficacy and safety of dosage-escalation of low-dosage esaxerenone added to a RAS inhibitor in hypertensive patients with type 2 diabetes and albuminuria: a single-arm, open-label study. Hypertens Res. 2019;42:1572–81.
Ito S, Itoh H, Rakugi H, Okuda Y, Iijima S. Antihypertensive effects and safety of esaxerenone in patients with moderate kidney dysfunction. Hypertens Res. 2021;44:489–97.
Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Kario K, Saito I, Kushiro T, et al. Morning home blood pressure is a strong predictor of coronary artery disease: the HONEST study. J Am Coll Cardiol. 2016;67:1519–27.
Rakugi H, Ito S, Itoh H, Okuda Y, Yamakawa S. Long-term phase 3 study of esaxerenone as mono or combination therapy with other antihypertensive drugs in patients with essential hypertension. Hypertens Res. 2019;42:1932–41.
Ito S, Kashihara N, Shikata K, et al. Esaxerenone (CS-3150) in patients with type 2 diabetes and microalbuminuria (ESAX-DN): phase 3 randomized controlled clinical trial. Clin J Am Soc Nephrol. 2020;15:1715–27.
Kobayashi Y, Haze T, Yano Y, et al. JPAS/JRAS Study Group. Associations between changes in plasma renin activity and aldosterone concentrations and changes in kidney function after treatment for primary aldosteronism. Kidney Int Rep. 2020;5:1291–7.
Saklayen MG, Gyebi LK, Tasosa J, Yap J. Effects of additive therapy with spironolactone on proteinuria in diabetic patients already on ACE inhibitor or ARB therapy: results of a randomized, placebo-controlled, double-blind, crossover trial. J Investig Med. 2008;56:714–9.
van den Meiracker AH, Baggen RG, Pauli S, et al. Spironolactone in type 2 diabetic nephropathy: effects on proteinuria, blood pressure and renal function. J Hypertens. 2006;24:2285–92.
Rossing K, Schjoedt KJ, Smidt UM, Boomsma F, Parving HH. Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: a randomized, double-masked, cross-over study. Diabetes Care. 2005;28:2106–12.
Epstein M, Williams GH, Weinberger M, et al. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2006;1:940–51.
Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–29.
Jiang X, Zhang Z, Li C, et al. Efficacy and safety of non-steroidal mineralocorticoid receptor antagonists in patients with chronic kidney disease and type 2 diabetes: a systemic review incorporating an indirect comparisons meta-analysis. Front Pharmacol. 2022;13:896947.
Lambers Heerspink HJ, Gansevoort RT. Albuminuria is an appropriate therapeutic target in patients with CKD: the pro view. Clin J Am Soc Nephrol. 2015;10:1079–88.
Lambers Heerspink HJ, Greene T, Tighiouart H, et al. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol. 2019;7:128–39.
Coresh J, Lambers Heerspink HJ, Sang Y, et al. Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019;7:115–27.
Ito S, Kashihara N, Shikata K, et al. Efficacy and safety of esaxerenone (CS-3150) in Japanese patients with type 2 diabetes and macroalbuminuria: a multicenter, single-arm, open-label phase III study. Clin Exp Nephrol. 2021;25:1070–8.
Pitt B, Filippatos G, Agarwal R, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385:2252–63.
Rakugi H, Yamakawa S, Sugimoto K. Management of hyperkalemia during treatment with mineralocorticoid receptor blockers: findings from esaxerenone. Hypertens Res. 2021;44:371–85.
Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685–93.
Edwards NC, Steeds RP, Chue CD, Stewart PM, Ferro CJ, Townend JN. The safety and tolerability of spironolactone in patients with mild to moderate chronic kidney disease. Br J Clin Pharm. 2012;73:447–54.
Ito S, Itoh H, Rakugi H, Okuda Y, Yoshimura M, Yamakawa S. Double-blind randomized phase 3 study comparing esaxerenone (CS-3150) and eplerenone in patients with essential hypertension (ESAX-HTN study). Hypertension. 2020;75:51–8.
Capolongo G, Capasso G, Viggiano D. A shared nephroprotective mechanism for renin-angiotensin-system inhibitors, sodium-glucose co-transporter 2 inhibitors, and vasopressin receptor antagonists: immunology meets hemodynamics. Int J Mol Sci. 2022;23:3915.
Acknowledgements
The authors would like to thank the participants in this study.
Funding
The EX-DKD study was supported by Daiichi Sankyo Co., Ltd., Tokyo, Japan, which was involved in the study design, planning of the data analysis, data interpretation, and development of the manuscript, but was not involved in the data management and statistical analysis. Data management and statistical analysis were performed by CMIC Co., Ltd. The Rapid Service and Open Access Fees were also funded by Daiichi Sankyo Co., Ltd.
Medical Writing and Editorial Assistance
Editorial assistance in the preparation of this article was provided by Michelle Belanger, MD, of Edanz (www.edanz.com) and was funded by Daiichi Sankyo Co., Ltd.
Author Contributions
Haruhito A. Uchida and Jun Wada contributed to the study design and planning of data analysis, conduct of the study, data interpretation, and writing/reviewing the manuscript. Hirofumi Nakajima, Masami Hashimoto, Akihiko Nakamura, Tomokazu Nunoue, Kazuharu Murakami, Takeshi Hosoya, and Kiichi Komoto contributed to the conduct of the study and writing/reviewing the manuscript. Takashi Taguchi and Kotaro Sugimoto contributed to the study design and planning of data analysis, data interpretation, and writing/reviewing the manuscript. Takaaki Akasaki contributed to the data interpretation and writing/reviewing the manuscript. Kazuhito Shiosakai contributed to the study design and planning of data analysis and writing/reviewing the manuscript. All authors read and approved the final manuscript.
Disclosures
Haruhito A. Uchida belongs to the Department of Chronic Kidney Disease and Cardiovascular Disease, which is endowed by Olba Healthcare Holdings Inc., Chugai Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., and Terumo Corporation, and has received speaker honoraria from Daiichi Sankyo Co., Ltd. Masami Hashimoto has received speaker honoraria from Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., and Astellas Pharma Inc. Takeshi Hosoya has received speaker honoraria from Eli Lilly Japan K.K., Taisho Pharmaceutical Co., Ltd., Fuji Yakuhin Co., Ltd., ZERIA Pharmaceutical Co., Ltd., and Kowa Co., Ltd. Takashi Taguchi, Takaaki Akasaka, Kazuhito Shiosakai, and Kotaro Sugimoto are employees of Daiichi Sankyo Co., Ltd. Jun Wada has received speaker honoraria from AstraZeneca K.K., Daiichi Sankyo Co., Ltd., Novartis Pharma K.K., Novo Nordisk Pharma Ltd., and Mitsubishi Tanabe Pharma Corp., and has received grant support from Astellas Pharma Inc., Baxter Ltd., Bayer Yakuhin, Ltd., Chugai Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Kyowa Kirin Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corp., and Teijin Pharma Ltd. Hirofumi Nakajima, Akihiko Nakamura, Tomokazu Nunoue, Kazuharu Murakami, and Kiichi Komoto have no conflicts of interest to disclose.
Compliance with Ethics Guidelines
The study protocol was approved by the Okayama University Certified Review Board (CRB6180001) and prospectively registered with the Japan Registry of Clinical Trials (jRCTs061190027; https://jrct.niph.go.jp/en-latest-detail/jRCTs061190027). The study was conducted in accordance with the principles of the Declaration of Helsinki 1964, and its later amendments, and the Clinical Trials Act in Japan. All patients provided written informed consent before enrollment.
Data availability
The anonymized data underlying the results presented in this manuscript may be made available to researchers upon submission of a reasonable request to the corresponding author. The decision to disclose the data will be made by the corresponding author and the funder, Daiichi Sankyo Co., Ltd. Data disclosure can be requested for 36 months from article publication.
Author information
Authors and Affiliations
Consortia
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
12325_2022_2294_MOESM1_ESM.pdf
Supplementary file1 (PDF 1047 KB) A Microsoft Word document contains study design (figure); changes in home and office SBP/DBP (figure); changes in eGFRcreat and serum potassium (figure); investigators list (table); dose characteristics (table); changes in BP (table); achievement of target BP levels (table); changes in UACR (table); biomarker (table); incidence of serum potassium level ≥ 5.5 and ≥ 6.0 mEq/L (table)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Uchida, H.A., Nakajima, H., Hashimoto, M. et al. Efficacy and Safety of Esaxerenone in Hypertensive Patients with Diabetic Kidney Disease: A Multicenter, Open-Label, Prospective Study. Adv Ther 39, 5158–5175 (2022). https://doi.org/10.1007/s12325-022-02294-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02294-z