Abstract
Background
Ankylosing spondylitis (AS) is a chronic inflammatory disease. Several proinflammatory cytokines produced by T helper 17 (Th17) cells are involved in the pathogenesis of AS. We performed a meta-analysis to determine the levels of Th17 cells and serum Th17-associated cytokines in patients with AS.
Methods
We determined the levels of Th17 cells and Th17 cytokines in patients with AS using data extracted from published articles retrieved from the PubMed, Embase, Web of Science, Cochrane Library, MEDLINE, Web of Knowledge, Clinical Trials.gov, and FDA.gov. databases. The effect estimates were pooled using a random-effects model. The review protocols were registered on PROSPERO (reference: CRD42021255741) and followed the PRISMA guideline.
Results
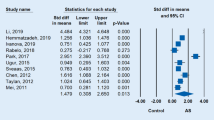
This meta-analysis included 138 studies. Compared to healthy controls (HCs), patients with AS had a higher proportion of Th17 cells (standardized mean difference [SMD] 2.23, 95% confidence interval [CI] 1.78–2.68; p < 0.001) and levels of proinflammatory cytokines, such as interleukin (IL)-17 (SMD 2.04, 95% CI 1.70–2.38; p < 0.001), IL-21 (SMD 1.77, 95% CI 0.95–2.59; p < 0.001), and IL-23 (SMD 1.11, 95% CI 0.78–1.44; p < 0.001). The subgroup analysis showed higher levels of IL-17+ Th17 cells among peripheral blood mononuclear cells (PBMCs) and CD4+ T cells in patients with AS compared to HCs (SMD 2.26, 95% CI 1.58–2.94 [p < 0.001] and SMD 1.61, 95% CI 0.55–2.67 [p = 0.003], respectively). Patients with AS had higher levels of CD4+IL-17+IFN-γ− Th17 in PBMCs and of CD4+CCR6+CCR4+Th17 in CD4+ T cells compared to HCs (SMD 1.85, 95% CI 1.06–2.64 [p < 0.001] and SMD 7.72, 95% CI 6.55–8.89 [p < 0.001], respectively). No significant differences were observed in the proportions of CD4+IL-17+IFN-γ− Th17 in CD4+ T cells and CD4+CCR6+CCR4+ Th17 in PBMCs (SMD − 0.11, 95% CI − 0.61 to 0.38 [p = 0.650] and SMD 1.32, 95% CI − 0.54 to 3.19 [p = 0.165], respectively). In addition, compared to stable AS, the levels of Th17 cells and IL-17 and IL-23 were significantly higher in active AS (SMD 1.58, 95% CI 0.30–2.85 [p = 0.016], SMD 3.52, 95% CI 0.72–6.33 [p = 0.014], and SMD 5.10, 95% CI 1.83–8.36 [p = 0.002], respectively).
Conclusions
The levels of Th17 cells and serum IL-17, IL-21, and IL-23 were higher in patients with AS than in HCs and, compared with stable AS, they increased more significantly in active AS. These results suggest that Th17 cells and Th17-related cytokines play major roles in AS pathogenesis and are an important target for treatment.










Similar content being viewed by others
References
Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390(10089):73–84.
Gratacos J, Collado A, Filella X, et al. Serum cytokines (IL-6, TNF-alpha, IL-1 beta and IFN-gamma) in ankylosing spondylitis: a close correlation between serum IL-6 and disease activity and severity. Br J Rheumatol. 1994;33(10):927–31.
Wendling D, Cedoz JP, Racadot E, Dumoulin G. Serum IL-17, BMP-7, and bone turnover markers in patients with ankylosing spondylitis. Joint Bone Spine. 2007;74(3):304–5.
Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60(6):1647–56.
Liu D, Liu B, Lin C, Gu J. Imbalance of peripheral lymphocyte subsets in patients with ankylosing spondylitis: a meta-analysis. Front Immunol. 2021;12: 696973.
Qian Y, Kang Z, Liu C, Li X. IL-17 signaling in host defense and inflammatory diseases. Cell Mol Immunol. 2010;7(5):328–33.
Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–41.
Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763–76.
Zygmunt B, Veldhoen M. T helper cell differentiation more than just cytokines. Adv Immunol. 2011;109:159–96.
Layh-Schmitt G, Colbert RA. The interleukin-23/interleukin-17 axis in spondyloarthritis. Curr Opin Rheumatol. 2008;20(4):392–7.
Yasuda K, Takeuchi Y, Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin Immunopathol. 2019;41(3):283–97.
Xie J, Wang Z, Wang W. Semaphorin 4D induces an imbalance of Th17/Treg cells by activating the aryl hydrocarbon receptor in ankylosing spondylitis. Front Immunol. 2020;11:2151.
Bautista-Caro MB, Arroyo-Villa I, Castillo-Gallego C, et al. Decreased Th17 and Th1 cells in the peripheral blood of patients with early non-radiographic axial spondyloarthritis: a marker of disease activity in HLA-B27(+) patients. Rheumatology (Oxford). 2013;52(2):352–62.
Ulrich J, Probst A, Anderton BH, Kahn J. Dementia of Alzheimer type (DAT)—a review of its morbid anatomy. Klin Wochenschr. 1986;64(3):103–14.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339: b2535.
Mingebach T, Kamp-Becker I, Christiansen H, Weber L. Meta-meta-analysis on the effectiveness of parent-based interventions for the treatment of child externalizing behavior problems. PLoS One. 2018;13(9): e0202855.
Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Matsuda A. Diabetic gangrene—physiopathology and management. Kango Gijutsu. 1979;25(16):105–15.
Akimzhanov AM, Yang XO, Dong C. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J Biol Chem. 2007;282(9):5969–72.
Wilson AS, Randall KL, Pettitt JA, et al. Neutrophil extracellular traps and their histones promote Th17 cell differentiation directly via TLR2. Nat Commun. 2022;13(1):528.
Marks KE, Rao DA. T peripheral helper cells in autoimmune diseases. Immunol Rev. 2022;307:191–202.
Yang J, Sundrud MS, Skepner J, Yamagata T. Targeting Th17 cells in autoimmune diseases. Trends Pharmacol Sci. 2014;35(10):493–500.
Sieper J, Poddubnyy D, Miossec P. The IL-23-IL-17 pathway as a therapeutic target in axial spondyloarthritis. Nat Rev Rheumatol. 2019;15(12):747–57.
Cao W, Wang X, Chen T, et al. The expression of notch/notch ligand, IL-35, IL-17, and Th17/Treg in preeclampsia. Dis Markers. 2015;2015: 316182.
McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity. 2019;50(4):892–906.
Lubberts E. The IL-23–IL-17 axis in inflammatory arthritis. Nat Rev Rheumatol. 2015;11(7):415–29.
Jethwa H, Bowness P. The interleukin (IL)-23/IL-17 axis in ankylosing spondylitis: new advances and potentials for treatment. Clin Exp Immunol. 2016;183(1):30–6.
Burkett PR, Meyer zu Horste G, Kuchroo VK. Pouring fuel on the fire: Th17 cells, the environment, and autoimmunity. J Clin Investig. 2015;125(6):2211–9.
Gravallese EM, Schett G. Effects of the IL-23–IL-17 pathway on bone in spondyloarthritis. Nat Rev Rheumatol. 2018;14(11):631–40.
Bridgewood C, Sharif K, Sherlock J, Watad A, McGonagle D. Interleukin-23 pathway at the enthesis: the emerging story of enthesitis in spondyloarthropathy. Immunol Rev. 2020;294(1):27–47.
McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382(9894):780–9.
Baeten D, Ostergaard M, Wei JC, et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann Rheum Dis. 2018;77(9):1295–302.
Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23–IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14(9):585–600.
McGonagle D, Watad A, Sharif K, Bridgewood C. Why inhibition of IL-23 lacked efficacy in ankylosing spondylitis. Front Immunol. 2021;12: 614255.
Siebert S, Millar NL, McInnes IB. Why did IL-23p19 inhibition fail in AS: a tale of tissues, trials or translation? Ann Rheum Dis. 2019;78(8):1015–8.
Zielinski CE, Mele F, Aschenbrenner D, et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484(7395):514–8.
Yang PT, Kasai H, Zhao LJ, Xiao WG, Tanabe F, Ito M. Increased CCR4 expression on circulating CD4(+) T cells in ankylosing spondylitis, rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Immunol. 2004;138(2):342–7.
Maeda S, Osaga S, Maeda T, et al. Circulating Th17.1 cells as candidate for the prediction of therapeutic response to abatacept in patients with rheumatoid arthritis: an exploratory research. PLoS One. 2019;14(11): e0215192.
Mease P, van den Bosch F. IL-23 and axial disease: do they come together? Rheumatology (Oxford). 2021;60(Suppl 4):iv28–33.
Acknowledgements
An earlier version of this study was presented as a poster in 23rd Asia-Pacific League of Associations for Rheumatology (https://onlinelibrary.wiley.com/doi/10.1111/1756-185X.14200).
Funding
This work was supported by the National Natural Science Foundation of China (No. 82001740). The journal’s Rapid Service Fee was supported by The Second Hospital of Shanxi Medical University.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
QYS designed the study. JWZ and TYZ did the database search and selected the data. QYS and JYY analyzed the data. QYS and JWZ wrote the manuscript. SS, RZ, JKD, SXZ, CHW, HYG contributed to manuscript revision, read the revised manuscript, and approved the submitted version.
Disclosures
None of the the authors reports any conflict of interest, financial or otherwise. Qin-Yi Su, Jing-Wen Zheng, Jing-Yuan Yang, Tong-Yuan Zhang, Shan Song, Rong Zhao, Jing-Kai Di, Sheng-Xiao Zhang, Cai-Hong Wang and Hui-Ying Gao have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data relevant to the study are included in the article or uploaded as online supplemental information. No additional data available.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, QY., Zheng, JW., Yang, JY. et al. Levels of Peripheral Th17 Cells and Th17-Related Cytokines in Patients with Ankylosing Spondylitis: A Meta-analysis. Adv Ther 39, 4423–4439 (2022). https://doi.org/10.1007/s12325-022-02240-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02240-z