Abstract
Introduction
Treatment persistence for anticoagulant therapy is important in preventing thromboembolism in nonvalvular atrial fibrillation (NVAF) patients. Understanding drug utilization pattern and treatment changes in oral anticoagulant (OAC) users may facilite better NVAF management. Thus, our study aimed to examine OAC treatment patterns preceding events leading to switch or discontinuation and medication adherence in Korean NVAF patients.
Methods
We conducted a drug utilization study on all Korean patients with atrial fibrillation (AF) newly prescribed OACs between July 2015 and November 2016 using the national claims data. We assessed treatment changes such as switching and discontinuation from index OAC and relevant events preceding the change and examined patient characteristics as predictors of changes that occurred among OAC users. Medication adherence was compared among OAC users by calculating the medication possession ratio (MPR).
Results
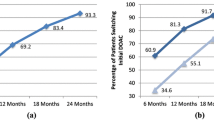
A total of 48,389 NVAF patients were identified who initiated OACs within the study period. Most initiated nonvitamin K antagonist oral anticoagulants (NOACs) (22% apixaban, 24% dabigatran, 37% rivaroxaban), and 18% initiated warfarin. The frequency of switch to another OAC was 8.8% for apixaban, 16.1% for dabigatran, 6.6% for rivaroxaban, and 19.1% for warfarin. The frequency of discontinuation was lower for apixaban (22.9%), dabigatran (26.3%), and rivaroxaban (25.7%) than warfarin (31.6%). Compared to warfarin, NOAC users were less likely to switch treatment. Thromboembolic event was the most common clinical event preceding switch from warfarin to NOAC and from NOAC to warfarin. Discontinuation of OAC was often preceded by a bleeding event. Patients who initiated apixaban showed significantly higher mean MPR compared to those on dabigatran and warfarin.
Conclusion
In real-world practice in Korea, we have observed treatment change to be common in OAC users. Our results indicate better medication adherence with NOACs than with warfarin. (ClinicalTrials.gov registration number NCT03572972).
Plain language summary
Anticoagulants are drugs that thin blood with the purpose of preventing thromboembolic disease (e.g., stroke), which is a disease occurring when a blood clot forms or blocks vessel. Maintaining treatment for anticoagulation is important to prevent stroke in atrial fibrillation (AF) patients. To understand current drug usage pattern and treatment changes related to oral anticoagulants (OAC) we examined OAC treatment patterns and preceding events that led to drug switch or stop and medication maintenance by Korean AF patients.
The study was conducted by utilizing the Korean national claims data from July 2015 to November 2016. All AF patients who newly started taking OAC were included in the analysis. In total, 48,389 patients were identified with most (83%) taking nonvitamin K antagonist oral anticoagulants (NOAC), which are newer generation blood thinners, including apixaban, dabigatran, and rivaroxaban, and 18% taking warfarin, the conventional blood thinner. Compared to warfarin, NOAC users were less likely to switch to other treatment. NOAC users discontinued the treatment less frequently than warfarin users. Thromboembolic events commonly preceded switch between OACs. Patients who stopped taking OACs were often confronted with a bleeding event before stopping treatment. Apixaban takers showed higher treatment persistence compared to dabigatran or warfarin users. In this study, we determined that treatment change is common in OAC-using patients. The results suggest that NOAC users may better adhere to treatment than warfarin users.






Similar content being viewed by others
References
Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51.
Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104.
Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92.
Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91.
Andrade JG, Aguilar M, Atzema C, et al. The 2020 Canadian cardiovascular society/Canadian heart rhythm society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol. 2020;36:1847–948.
Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498.
January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. 2019;140:e125–51.
Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638-45.e4.
Huisman MV, Rothman KJ, Paquette M, et al. The changing landscape for stroke prevention in AF: findings from the GLORIA-AF registry phase 2. J Am Coll Cardiol. 2017;69:777–85.
Baker CL, Dhamane AD, Mardekian J, et al. Comparison of drug switching and discontinuation rates in patients with nonvalvular atrial fibrillation treated with direct oral anticoagulants in the United States. Adv Ther. 2019;36:162–74.
Beyer-Westendorf J, Ehlken B, Evers T. Real-world persistence and adherence to oral anticoagulation for stroke risk reduction in patients with atrial fibrillation. Europace. 2016;18:1150–7.
Forslund T, Wettermark B, Hjemdahl P. Comparison of treatment persistence with different oral anticoagulants in patients with atrial fibrillation. Eur J Clin Pharmacol. 2016;72:329–38.
Hellfritzsch M, Grove EL, Husted SE, et al. Clinical events preceding switching and discontinuation of oral anticoagulant treatment in patients with atrial fibrillation. Europace. 2017;19:1091–5.
Hohnloser SH, Basic E, Nabauer M. Changes in oral anticoagulation therapy over one year in 51,000 atrial fibrillation patients at risk for stroke: a practice-derived study. Thromb Haemost. 2019;119:882–93.
Johnson ME, Lefèvre C, Collings SL, et al. Early real-world evidence of persistence on oral anticoagulants for stroke prevention in non-valvular atrial fibrillation: a cohort study in UK primary care. BMJ Open. 2016; 6:e011471.
Lip GYH, Pan X, Kamble S, et al. Discontinuation risk comparison among 'real-world' newly anticoagulated atrial fibrillation patients: Apixaban, warfarin, dabigatran, or rivaroxaban. PloS One. 2018; 13:e0195950.
Manzoor BS, Walton SM, Sharp LK, Galanter WL, Lee TA, Nutescu EA. High number of newly initiated direct oral anticoagulant users switch to alternate anticoagulant therapy. J Thromb Thrombolysis. 2017;44:435–41.
Martinez C, Katholing A, Wallenhorst C, Freedman SB. Therapy persistence in newly diagnosed non-valvular atrial fibrillation treated with warfarin or NOAC. A cohort study Thromb Haemost. 2016;115:31–9.
Ruigómez A, Vora P, Balabanova Y, et al. Discontinuation of non-Vitamin K antagonist oral anticoagulants in patients with non-valvular atrial fibrillation: a population-based cohort study using primary care data from The Health Improvement Network in the UK. BMJ Open. 2019; 9:e031342.
Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost. 2014;111:789–97.
Lip GY, Wang KL, Chiang CE. Non-vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in Asian patients with atrial fibrillation: time for a reappraisal. Int J Cardiol. 2015;180:246–54.
Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of korea health insurance review and assessment (hira) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017;32:718–28.
Kim TH, Yang PS, Uhm JS, et al. CHA(2)DS(2)-VASc Score (Congestive Heart Failure, Hypertension, Age ≥75 [Doubled], Diabetes Mellitus, Prior Stroke or Transient Ischemic Attack [Doubled], Vascular Disease, Age 65–74, Female) for Stroke in Asian Patients With Atrial Fibrillation: A Korean Nationwide Sample Cohort Study. Stroke. 2017;48:1524–30.
Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3–12.
Kim H, Kim TH, Cha MJ, et al. A prospective survey of atrial fibrillation management for real-world guideline adherence: comparison study of drugs for symptom control and complication prEvention of atrial fibrillation (CODE-AF) registry. Korean Circ J. 2017;47:877–87.
Yu HT, Yang PS, Hwang J, et al. Social inequalities of oral anticoagulation after the introduction of non-vitamin K antagonists in patients with atrial fibrillation. Korean Circ J. 2020;50:267–77.
Cha MJ, Choi EK, Han KD, et al. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in asian patients with atrial fibrillation. Stroke. 2017;48:3040–8.
Cho MS, Yun JE, Park JJ, et al. Outcomes After Use of Standard- and Low-Dose Non-Vitamin K Oral Anticoagulants in Asian Patients With Atrial Fibrillation. Stroke. 2018:STROKEAHA118023093.
Lee SR, Choi EK, Han KD, Jung JH, Oh S, Lip GYH. Edoxaban in asian patients with atrial fibrillation: effectiveness and safety. J Am Coll Cardiol. 2018;72:838–53.
Del-Carpio Munoz F, Gharacholou SM, Munger TM, et al. Meta-analysis of renal function on the safety and efficacy of novel oral anticoagulants for atrial fibrillation. Am J Cardiol. 2016;117:69–75.
Lee SR, Choi EK, Kwon S, et al. Oral anticoagulation in asian patients with atrial fibrillation and a history of intracranial hemorrhage. Stroke. 2020;51:416–23.
Nielsen PB, Skjøth F, Søgaard M, Kjældgaard JN, Lip GYH, Larsen TB. Non-Vitamin K antagonist oral anticoagulants versus warfarin in atrial fibrillation patients with intracerebral hemorrhage. Stroke. 2019;50:939–46.
Wetmore JB, Roetker NS, Yan H, Reyes JL, Herzog CA. Direct-acting oral anticoagulants versus warfarin in medicare patients with chronic kidney disease and atrial fibrillation. Stroke. 2020;51:2364–73.
Baker CL, Dhamane AD, Rajpura J, et al. Switching to another oral anticoagulant and drug discontinuation among elderly patients with nonvalvular atrial fibrillation treated with different direct oral anticoagulants. Clin Appl Thromb Hemost. 2019;25:1076029619870249.
Sørensen R, Jamie Nielsen B, Langtved Pallisgaard J, Ji-Young Lee C, Torp-Pedersen C. Adherence with oral anticoagulation in non-valvular atrial fibrillation: a comparison of vitamin K antagonists and non-vitamin K antagonists. Eur Heart J Cardiovasc Pharmacother. 2017;3:151–6.
Fosbøl EL, Vinding NE, Lamberts M, et al. Shifting to a non-vitamin K antagonist oral anticoagulation agent from vitamin K antagonist in atrial fibrillation. Europace. 2018;20:e78-86.
Sciria CT, Maddox TM, Marzec L, et al. Switching warfarin to direct oral anticoagulants in atrial fibrillation: insights from the NCDR PINNACLE registry. Clin Cardiol. 2020;43:743–51.
Gallù M, Marrone G, Legramante JM, De Lorenzo A, Di Daniele N, Noce A. Female sex as a thromboembolic risk factor in the era of nonvitamin K antagonist oral anticoagulants. Cardiovasc Ther. 2020;2020:1743927.
Kim H, Lee YS, Kim TH, et al. A prospective survey of the persistence of warfarin or NOAC in nonvalvular atrial fibrillation: a COmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation (CODE-AF). Korean J Intern Med. 2020;35:99–108.
Beyer-Westendorf J, Förster K, Ebertz F, et al. Drug persistence with rivaroxaban therapy in atrial fibrillation patients-results from the Dresden non-interventional oral anticoagulation registry. Europace. 2015;17:530–8.
Beyer-Westendorf J, Gelbricht V, Förster K, et al. Safety of switching from vitamin K antagonists to dabigatran or rivaroxaban in daily care–results from the Dresden NOAC registry. Br J Clin Pharmacol. 2014;78:908–17.
Salmasi S, Loewen PS, Tandun R, Andrade JG, De Vera MA. Adherence to oral anticoagulants among patients with atrial fibrillation: a systematic review and meta-analysis of observational studies. BMJ Open 2020; 10:e034778.
Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–74.
Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25:2303–10.
Ozaki AF, Choi AS, Le QT, et al. Real-World Adherence and Persistence to Direct Oral Anticoagulants in Patients With Atrial Fibrillation: A Systematic Review and Meta-Analysis. Circ Cardiovasc Qual Outcomes. 2020; 13:e005969.
Yao X, Abraham NS, Alexander GC, et al. Effect of Adherence to Oral Anticoagulants on Risk of Stroke and Major Bleeding Among Patients With Atrial Fibrillation. J Am Heart Assoc. 2016; 5:e003074.
Acknowledgements
Funding
This study was sponsored by Pfizer and Bristol-Myers Squibb; the Journal’s Rapid Service Fee was funded by Pfizer.
Medical Writing, Editorial, and Other Assistance
Editorial support on English language was provided by Enago (www.enago.co.kr) and was funded by Pfizer and Bristol Myers Squibb.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: Sola Han, Yoo-Jung Park, and Hae Sun Suh; Methodology: Sola Han and Hae Sun Suh; Investigation: Myong-Yong Lee, Sola Han, Oh Young Bang, Young-Keun On, Sung-Won Jang, Seongwook Han, Hae Sun Suh and Young-Hoon Kim; Data curation and Formal analysis: Sola Han and Hae Sun Suh; Writing—original draft preparation: Myong-Yong Lee, Sola Han, Jaeyun Ryu, Yoo-Jung Park and Hae Sun Suh; Writing—review and editing: All authors; Project administration: Jaeyun Ryu, Yoo-Jung Park and Seongsik Kang; Supervision: Yoo-Jung Park, Seongsik Kang, Hae Sun Suh and Young-Hoon Kim.
Prior Presentation
The abstract of this study was presented at American Heart Association 2018 Congress in Chicago, IL, USA, in November 2018.
Disclosures
Authors Jaeyun Ryu, Yoo-Jung Park, and Seongsik Kang are full-time employees and stockholders of Pfizer Inc. Authors Myung-Yong Lee, Sola Han, Oh Young Bang, Young Keun On, Sung-Won Jang, Seongwook Han, Hae Sun Suh, and Young-Hoon Kim are paid consultants for Pfizer Korea Ltd.
Compliance with Ethics Guidelines
Ethical clearance for the study was exempted by the institutional review board at Pusan National University (PNU IRB/2016_137_HR).
Data Availability
The study data were extracted and analyzed from the Korean Health Insurance Review & Assessment Service (HIRA) claims database, and additional data may be obtained from third party (with appropriate authorization approval) but are not publicly available.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, MY., Han, S., Bang, O.Y. et al. Drug Utilization Pattern of Oral Anticoagulants in Patients with Atrial Fibrillation: A Nationwide Population-Based Study in Korea. Adv Ther 39, 3112–3130 (2022). https://doi.org/10.1007/s12325-022-02151-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02151-z