Abstract
Introduction
Patients diagnosed with cancer have an increased risk both for myelodysplastic syndromes (MDS) and for acute myeloid leukemia (AML) following treatment.
Methods
Using SEER-Medicare data, we selected patients aged 66 years and older who completed systemic therapy between 2002 and 2014 for breast (stage I–III), lung (stage I–III), or prostate (stage I–IV) cancer. For each cancer, we estimated the risk of a composite endpoint of MDS or AML in patients receiving granulocyte colony-stimulating factor (G-CSF) vs. not.
Results
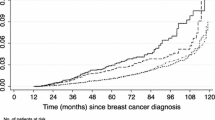
The 10-year cumulative risk difference (granulocyte colony-stimulating factor [G-CSF] − no G-CSF) for MDS-AML was 0.45% (95% CI 0.13–0.77%) in breast cancer and 0.39% (95% CI 0.15–0.62%) in lung cancer. G-CSF use was associated with a hazard ratio of 1.60 (95% CI 1.07–2.40) in breast cancer and 1.50 (95% CI 0.99–2.29) in lung cancer. Filgrastim use was associated with a hazard ratio of 1.01 (95% CI 1.00–1.03) per administration in breast cancer and 1.02 (95% CI 0.99–1.05) per administration in lung cancer. Pegfilgrastim was associated with a hazard ratio of 1.08 (95% CI 1.01–1.15) per administration in breast cancer and 1.12 (95% CI 1.00–1.25) per administration in lung cancer. Analyses in prostate cancer were limited because of the low number of events.
Conclusions
The use of G-CSF in patients diagnosed with breast and lung cancer is associated with an increased risk of MDS-AML. However, the MDS-AML absolute risk difference is very low.

Similar content being viewed by others
References
Lyman GH, Dale DC, Wolff DA, et al. Acute myeloid leukemia or myelodysplastic syndrome in randomized controlled clinical trials of cancer chemotherapy with granulocyte colony-stimulating factor: a systematic review. J Clin Oncol. 2010;28(17):2914–24. https://doi.org/10.1200/JCO.2009.25.8723.
Calip GS, Malmgren JA, Lee WJ, Schwartz SM, Kaplan HG. Myelodysplastic syndrome and acute myeloid leukemia following adjuvant chemotherapy with and without granulocyte colony-stimulating factors for breast cancer. Breast Cancer Res Treat. 2015;154(1):133–43. https://doi.org/10.1007/s10549-015-3590-1.
Castro GA, Church A, Pechet L, Snyder LM. Leukemia after chemotherapy of Hodgkin’s disease. N Engl J Med. 1973;289(2):103–4. https://doi.org/10.1056/nejm197307122890215.
Preisler HD, Lyman GH. Acute myelogenous leukemia subsequent to therapy for a different neoplasm: clinical features and response to therapy. Am J Hematol. 1977;3(3):209–18. https://doi.org/10.1002/ajh.2830030301.
Kaplan HG, Malmgren JA, Atwood MK. Increased incidence of myelodysplastic syndrome and acute myeloid leukemia following breast cancer treatment with radiation alone or combined with chemotherapy: a registry cohort analysis 1990–2005. BMC Cancer. 2011;11:260. https://doi.org/10.1186/1471-2407-11-260.
Larson RA. Is secondary leukemia an independent poor prognostic factor in acute myeloid leukemia? Best Pract Res Clin Haematol. 2007;20(1):29–37. https://doi.org/10.1016/j.beha.2006.10.006.
Godley LA, Larson RA. Therapy-related myeloid leukemia. Semin Oncol. 2008;35(4):418–29. https://doi.org/10.1053/j.seminoncol.2008.04.012.
Praga C, Bergh J, Bliss J, et al. Risk of acute myeloid leukemia and myelodysplastic syndrome in trials of adjuvant epirubicin for early breast cancer: correlation with doses of epirubicin and cyclophosphamide. J Clin Oncol. 2005;23(18):4179–91. https://doi.org/10.1200/JCO.2005.05.029.
Smith RE, Bryant J, DeCillis A, Anderson S. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the national surgical adjuvant breast and bowel project experience. J Clin Oncol. 2003;21(7):1195–204. https://doi.org/10.1200/JCO.2003.03.114.
Patt DA, Duan Z, Fang S, Hortobagyi GN, Giordano SH. Acute myeloid leukemia after adjuvant breast cancer therapy in older women: understanding risk. J Clin Oncol. 2007;25(25):3871–6. https://doi.org/10.1200/JCO.2007.12.0832.
Weycker D, Bensink M, Lonshteyn A, Doroff R, Chandler D. Risk of chemotherapy-induced febrile neutropenia by day of pegfilgrastim prophylaxis in US clinical practice from 2010 to 2015. Curr Med Res Opin. 2017;33(12):2107–13. https://doi.org/10.1080/03007995.2017.1386858.
Touw IP, Bontenbal M. Granulocyte colony-stimulating factor: key (f)actor or innocent bystander in the development of secondary myeloid malignancy? J Natl Cancer Inst. 2007;99(3):183–6. https://doi.org/10.1093/jnci/djk057.
Dong F, Brynes RK, Tidow N, Welte K, Löwenberg B, Touw IP. Mutations in the gene for the granulocyte colony-stimulating–factor receptor in patients with acute myeloid leukemia preceded by severe congenital neutropenia. N Engl J Med. 1995;333(8):487–93. https://doi.org/10.1056/NEJM199508243330804.
Voso MT, Falconi G, Fabiani E. What’s new in the pathogenesis and treatment of therapy-related myeloid neoplasms. Blood. 2021;138(9):749–57. https://doi.org/10.1182/blood.2021010764.
Pich O, Cortes-Bullich A, Muiños F, Pratcorona M, Gonzalez-Perez A, Lopez-Bigas N. The evolution of hematopoietic cells under cancer therapy. Nat Commun. 2021. https://doi.org/10.1038/s41467-021-24858-3.
Hershman D, Neugut AI, Jacobson JS, et al. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst. 2007;99(3):196–205. https://doi.org/10.1093/jnci/djk028.
Food and Drug Administration. Filgrastim [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103353s5183lbl.pdf. Accessed 13 June 2018.
Food and Drug Administration. Pegfilgrastim [prescribing information]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125031s180lbl.pdf. Accessed 13 June 2018.
Molineux G, Foote MA, Arvedson T. Twenty years of G-CSF: clinical and nonclinical discoveries. Basel: Springer; 2012.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. https://doi.org/10.3322/caac.21332.
Wang L, Barron R, Baser O, Langeberg WJ, Dale DC. Cancer chemotherapy treatment patterns and febrile neutropenia in the US Veterans Health Administration. Value Health. 2014;17(6):739–43. https://doi.org/10.1016/j.jval.2014.06.009.
Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–87. https://doi.org/10.1016/j.ejca.2018.07.005.
Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med care. 2002;40(8 Suppl):IV-3–18. https://doi.org/10.1097/01.MLR.0000020942.47004.03.
Enewold L, Parsons H, Zhao L, et al. Updated overview of the SEER-medicare data: enhanced content and applications. J Natl Cancer Inst Monogr. 2020;2020(55):3–13. https://doi.org/10.1093/jncimonographs/lgz029.
Suissa S. Immortal time bias in pharmacoepidemiology. Am J Epidemiol. 2008;167(4):492–9. https://doi.org/10.1093/aje/kwm324.
Sargramostim Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103362s5240lbl.pdf. Accessed 15 Feb 2021
National Cancer Institute. Observational Research in Oncology Toolbox. https://seer.cancer.gov/oncologytoolbox/. Accessed 17 Oct 2019.
Noone AM, Lund JL, Mariotto A, et al. Comparison of SEER treatment data with medicare claims. Med Care. 2016;54(9):e55-64. https://doi.org/10.1097/MLR.0000000000000073.
Paravati AJ, Boero IJ, Triplett DP, et al. Variation in the cost of radiation therapy among medicare patients with cancer. J Oncol Pract. 2015;11(5):403–9. https://doi.org/10.1200/jop.2015.005694.
Bleicher RJ, Ruth K, Sigurdson ER, et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2(3):330–9. https://doi.org/10.1001/jamaoncol.2015.4508.
Beebe-Dimmer JL, Ruterbusch JJ, Cooney KA, et al. Racial differences in patterns of treatment among men diagnosed with de novo advanced prostate cancer: a SEER-Medicare investigation. Cancer Med. 2019;8(6):3325–35. https://doi.org/10.1002/cam4.2092.
Nadpara PA, Madhavan SS, Tworek C. Disparities in lung cancer care and outcomes among elderly in a medically underserved state population—a cancer registry-linked database study. Popul Health Manag. 2016;19(2):109–19. https://doi.org/10.1089/pop.2015.0027.
Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17(8):584–90. https://doi.org/10.1016/j.annepidem.2007.03.011.
Cogle CR, Craig BM, Rollison DE, List AF. Incidence of the myelodysplastic syndromes using a novel claims-based algorithm: high number of uncaptured cases by cancer registries. Blood. 2011;117(26):7121–5. https://doi.org/10.1182/blood-2011-02-337964.
Craig BM, Rollison DE, List AF, Cogle CR. Underreporting of myeloid malignancies by United States cancer registries. Cancer Epidemiol Biomark Prev. 2012;21(3):474–81. https://doi.org/10.1158/1055-9965.EPI-11-1087.
Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–56. https://doi.org/10.1093/aje/kwp107.
Comprehensive R Archive Network. Vienna: R Foundation for Statistical Computing.
Naeim A, Henk HJ, Becker L, et al. Pegfilgrastim prophylaxis is associated with a lower risk of hospitalization of cancer patients than filgrastim prophylaxis: a retrospective United States claims analysis of granulocyte colony-stimulating factors (G-CSF). BMC Cancer. 2013;13:11. https://doi.org/10.1186/1471-2407-13-11.
Gawade PL, Li S, Henry D, et al. Patterns of granulocyte colony-stimulating factor prophylaxis in patients with cancer receiving myelosuppressive chemotherapy. Supportive Care Cancer. 2020. https://doi.org/10.1007/s00520-020-05295-2.
Westreich D, Greenland S. The Table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177(4):292–8. https://doi.org/10.1093/aje/kws412.
Acknowledgements
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
Funding
Outcomes Insights, Inc was funded by Amgen to conduct this study. Amgen also paid the journal’s Rapid Service fees. Prior to conducting the research, Outcomes Insights, Inc. was permitted to publish the results of this study by Amgen, Inc., which is a requirement in the data use agreement with the National Cancer Institute.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the conception, study design, and protocol development. Mark Danese conducted the data analyses. Mark Danese and Jennifer Schenfeld were responsible for creating the initial draft. All authors were involved in revising the initial draft and approving the final version. All authors agree to be accountable for the integrity of the research.
Disclosures
At the time this research was conducted Jennifer Schenfeld, Jaime Shaw, Prasad Gawade, Akhila Balasubramanian, Michael Kelsh, and Rohini K. Hernandez were Amgen employees and Amgen stockholders. Gary Lyman served as a Principal Investigator on an institutional grant from Amgen, Inc. and received speaking or consulting fees from G1 Therapeutics, Partners Healthcare, BeyondSpring, Squibb (institutional), Sandoz, Merck, Jazz, Kallyope, and TEVA. Mark Danese was an owner of Outcomes Insights, Inc. Outcomes Insights, Inc. received research funding to conduct this study, including funding for developing the manuscript. Outcomes Insights, Inc. has also received consulting fees from Amgen related to methods for observational research. Outcomes Insights, Inc. provided research and consulting services in oncology to Bristol Myers Squibb, Seattle Genetics, Boston Scientific, Mirati, EMD Serono, and Taiho.
Compliance with Ethics Guidelines
The study protocol was reviewed by Advarra and received an exemption determination on 24 May 2019. The authors received permission to access and use the USRDS data from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Data Availability
The data sets generated for the current study are not publicly available to ensure patient privacy, as required by the data use agreement with the National Cancer Institute
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Danese, M.D., Schenfeld, J., Shaw, J. et al. Association Between Granulocyte Colony-Stimulating Factor (G-CSF) Use and Myelodysplastic Syndrome (MDS) or Acute Myeloid Leukemia (AML) Among Elderly Patients with Breast, Lung, or Prostate Cancer. Adv Ther 39, 2778–2795 (2022). https://doi.org/10.1007/s12325-022-02141-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02141-1