Abstract
Introduction
To date, there are limited real-world studies published on the use of infliximab-dyyb, a biosimilar to reference product (RP) infliximab approved for the treatment of moderate to severe inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC) in North America. This study examined utilization patterns and the effects of infliximab-dyyb on clinical outcomes, patient-reported outcomes (PROs), and healthcare resource use (HCRU) in IBD patients in a real-world setting.
Methods
In this prospective, observational study, adult IBD patients in the US and Canada were recruited to initiate treatment with infliximab-dyyb and followed for 12 months. Patients included biologic-naïve users of infliximab-dyyb and patients switching from RP infliximab or other biologics to infliximab-dyyb. Partial Mayo (pMAYO) and Harvey Bradshaw Index (HBI) scores measured clinical outcomes for the UC and CD cohorts, respectively. Key PRO measures included the SIBDQ, EQ-VAS, and psychological outcomes. In addition, work productivity, HCRU, and adverse events (AEs) were assessed.
Results
A total of 67 CD and 48 UC patients were enrolled (51% female; mean age 44 years; 87% Caucasian; mean BMI 27.9). Thirty-nine patients were biologic-naïve, 57 switched from RP infliximab, and 19 switched from other biologics. Among UC biologic-naïve users, pMAYO decreased from 5.67 to 1.09 (p < 0.0001) and the remission rate increased from 5.6 to 90.9% (p = 0.0015). For UC patients switching from RP infliximab, pMAYO decreased from 1.38 to 0.29 (p = 0.0103). For CD biologic-naïve users, HBI scores and remission rates did not significantly change. The scores on all the PROs significantly improved from baseline to 12 months. A total of 22 AEs occurred consistent with the known AE profile for infliximab.
Conclusions
Clinical outcomes among biologic-naïve users of infliximab-dyyb improved for UC and were maintained for CD patients. Biologic-naïve users of infliximab-dyyb showed significant improvements in PROs. Patients switching from RP infliximab to infliximab-dyyb maintained their clinical outcomes and PROs.
Trial Registration
ClinicalTrials.gov Registration Number: NCT03801928 (February 23, 2018).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The approval of infliximab-dyyb for use in Crohn’s disease (CD) and ulcerative colitis (UC) was granted based on extrapolation. |
To date, only limited real-world data have been published on the use of infliximab-dyyb and the clinical and patient-reported outcomes in inflammatory bowel disease (IBD) patients treated with infliximab-dyyb in the North American population. |
In this prospective, observational study, we evaluated real-world clinical outcomes, patient-reported outcomes, and healthcare resource utilization associated with the use of infliximab-dyyb to treat inflammatory bowel disease (IBD) among biologic-naïve patients and patients switching from reference product (RP) infliximab or other biologics. |
What was learned from the study? |
Among biologic-naïve patients, clinical outcomes improved significantly for UC patients and were maintained for CD ones. |
Consistent with findings across other immunological diseases, our study found that patients who switched from RP infliximab to infliximab-dyyb maintained clinical outcomes and remission status. |
Patient-reported quality-of-life and work productivity outcomes improved among biologic-naïve patients and were maintained for patients switched from RP infliximab. |
Although the number of patients in this study is small and direct comparisons cannot be made, adverse events occurred at a rate consistent with the known adverse event profile for RP infliximab. |
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are collectively known as inflammatory bowel disease (IBD) [1]. Both conditions are characterized by chronic inflammation of the bowel, a relapsing and remitting clinical course, lifelong medication use, and often significant morbidity.
The advent of biologics, such as infliximab, in the treatment of IBD has progressively changed the therapeutic landscape in this disease. Several studies of infliximab have demonstrated a benefit for disease outcome and control, a decrease in hospitalizations, and improvements in quality of life [2,3,4,5]. Despite the clinical benefits of infliximab for IBD, cost remains a concern; therefore, biosimilars that are less expensive than reference product (RP) biologics but have similar effectiveness have been of high interest to payers and managed care organizations.[6].
Biosimilars are biological medicines that were developed to be highly similar to originator or reference medicines, and to offer the potential of lower treatment cost [7]. The introduction of biosimilars offers an opportunity for increased patient access and decreased health expenditures across a number of immunologic indications. Biosimilar infliximab-dyyb (also known as CT-P13, branded as Inflectra®) was approved in the US and Canada in 2016 for IBD as a biosimilar to RP infliximab (branded as Remicade®) based on the concept of extrapolation [8].
Recently, a few studies outside North America have compared safety outcomes, such as hepatitis and tuberculosis infections, and the pharmacokinetic profile of infliximab biosimilar and the originator in the real-world IBD population. These studies found that infliximab biosimilar had similar safety and pharmacokinetic profile in real-world settings [9, 10]. Additional real-world studies from outside North America, where approval for infliximab-dyyb for IBD occurred before 2016, suggest no clinically meaningful differences in safety and effectiveness when patients either remain on RP infliximab or switch to an infliximab biosimilar [11,12,13,14,15,16,17].
To date, only limited real-world data have been published on the use of infliximab-dyyb, and the clinical and patient-reported outcomes in IBD patients treated with infliximab-dyyb, in the North American population. Post-approval non-interventional studies evaluating comparative outcomes can play a key role in building a real-world evidence base to help inform clinical practices and policy decisions [18]. Therefore, the goal of this study was to understand real-world treatment patterns of infliximab-dyyb and to assess its effects on clinical outcomes, patient-reported outcomes (PROs), and healthcare resource use in adult UC and CD patients treated with infliximab-dyyb in a real-world North American setting.
Methods
Study Design and Participants
This was a prospective observational study performed in 24 sites across the US and Canada. A geographically dispersed group of gastroenterologists in these countries recruited study patients. Physician inclusion and exclusion criteria are described below.
Physician inclusion criteria
-
Is certified to practice in the US or Canada;
-
Agrees to study rules including resolution of data queries, including missing data;
-
Routinely uses standard laboratory testing to monitor patient health;
-
Has access to certified laboratory for basic laboratory testing;
-
Can make available medical records and proper documentation for patients.
Physician exclusion criteria
-
Is unwilling or unable to follow study procedures;
-
Is unwilling to prescribe biosimilars.
Physicians recruited adult (≥ 18 years) patients initiating treatment with infliximab-dyyb for IBD (CD or UC) between February 2018 and February 2019. Patients were enrolled within 2 weeks of their first infusion with infliximab-dyyb. Patient inclusion and exclusion criteria are described below.
Patient inclusion criteria
-
Has a confirmed diagnosis of UC or CD;
-
Has evidence of a personally signed and dated informed consent document indicating that they have been informed of all pertinent aspects of the study;
-
Is eligible to receive infliximab-dyyb for the treatment of their disease per approved drug label (patients with fistula or stoma are eligible).
Patient exclusion criteria (any of the below)
-
Is less than 18 years old at the time of consent;
-
Previously failed treatment with RP infliximab or infliximab-dyyb;
-
Has reported contraindications for RP infliximab or infliximab-dyyb;
-
Has a known hypersensitivity (including severe, acute infusion reactions) to infliximab, its excipients, or other murine proteins;
-
Has difficulty reading or understanding the study consent or questionnaires.
Recruited individuals included IBD patients with no previous biologics use (biologic-naïve users), IBD patients switching from RP infliximab to infliximab-dyyb, and IBD patients switching to infliximab-dyyb from other biologics. Enrolled patients were followed prospectively for 12 months after initiating infliximab-dyyb treatment. There were no protocol-required medical procedures for this study.
As this was an observational study, the decision to treat a patient with infliximab-dyyb was made prior to that patient’s enrollment. Recruited physicians and/or their assigned staff were responsible for: patient identification, qualification, and selection; patient interviews; exam recording; data abstraction; and completion of patient case report forms. Study protocol and informed consent were reviewed and approved by the institutional review board at each site. Written informed consent was obtained from all patients enrolled in the study. Full details of institutional review boards that approved the study, along with relevant reference numbers, can be found in the Supplementary Material.
Study Measures
At baseline (the time of initiating infliximab-dyyb treatment), patient demographics (sex, age, race/ethnicity, insurance status) and clinical characteristics [body mass index (BMI), Charlson Comorbidity Index score, IBD type, duration of disease, and reason for treatment initiation] were recorded. Information about drug utilization patterns, clinical outcomes, PROs, and healthcare resource use was collected at infusion visits corresponding with the baseline visit and the 3-month, 6-month, and 12-month post-enrollment follow-up visits.
To describe infliximab-dyyb utilization patterns, the study assessed patients’ history of previous biologics use, patients’ reasons for initiating infliximab-dyyb, and discontinuation rates.
Clinical endpoints included disease remission status and disease response to treatment, as defined in disease-specific composite measures. In CD patients, disease remission was defined as a Harvey-Bradshaw Index (HBI) score of < 5 and response to treatment was defined as a reduction from baseline HBI ≥ 3 points. In UC patients, disease remission was defined as a Partial Mayo Score (pMAYO) score of < 3, and response to treatment was defined as a reduction from baseline pMAYO ≥ 3 points.
PROs measures were the Short Inflammatory Bowel Disease Questionnaire (SIBDQ); the EuroQol Visual Analogue Scale (EQ-VAS), the Treatment Satisfaction Questionnaire for Medication (TSQM), the Work Productivity and Activity Impairment (WPAI) questionnaire, and, to assess psychological outcomes, the General Anxiety Disorder-7 (GAD-7) questionnaire and the Patient Health Questionnaire-8 (PHQ-8; for depression).
Healthcare resource use measurements included the presence of IBD-related hospitalizations, presence of emergency department (ED) visits, and number of gastroenterologist visits. Visits recorded as part of resource use excluded visits to receive infliximab-dyyb infusions.
Adverse events (AEs) were monitored from each patient’s first infusion of infliximab-dyyb until their last follow-up visit. Enrolling physicians classified AEs as related to or unrelated to study treatment.
Statistical Analysis
Baseline demographic and clinical characteristics of biologic-naïve patients, patients switching from RP infliximab, and patients switching from other biologics were compared using the chi-square test or Fisher’s exact test (in case of a small sample size) for categorical variables (e.g., gender). The analysis of variance (ANOVA) or the Wilcoxon rank sum test was used to compare continuous variables (e.g., BMI).
Changes in outcomes over time from baseline were calculated using a mixed model for repeated measures (MMRM) for continuous outcomes and a generalized estimating equation (GEE) for categorical outcomes, accounting for the repeated nature of the data. Given the overall sample size, all analytical models were bivariate in nature, which included a specific outcome of interest as a dependent variable and a study visit as an independent variable. All analyses were conducted at an α level of 0.05 using SAS v.9.4. (SAS Institute, Cary, NC, USA.)
Results
From February 2018 to February 2020, 115 IBD patients (67 CD and 48 UC) initiated infliximab-dyyb treatment and were followed for 12 months (Fig. 1). Of 115 patients who completed the baseline visit (visit 1), 109 completed the 3-month visit (2), 99 completed the 6-month visit (3), and 84 completed the 12-month visit (4). Among the CD cohort, 20 (29.9%) had B1 (non-stricturing, non-penetrating disease). Among the UC cohort, 28 (58.3%) had E3 extensive disease. Overall, 24 (20.9%) patients had a history of IBD-related surgery. A total of 66 (57.3%) patients received endoscopy at baseline (Table 1), and 37 had endoscopy during the follow-up period. Of 66 patients, 60 patients (90.9%) received colonoscopy, and 6 (9.1%) received sigmoidoscopy. The most common reasons for endoscopy were routine surveillance (n = 22; 33.3%), diagnosis (n = 18; 27%), and assessment for disease activity (n = 22; 33.3%).
Infliximab-dyyb Utilization Patterns
Of 115 patients, 39 were biologic naïve, 57 were switched from RP infliximab, and 19 were switched from other biologics. Patient demographics are summarized in Table 1. There were no statistically significant demographic differences between groups except in BMI.
In patients switching from RP infliximab, the majority (80.4%) of patient reasons for infliximab-dyyb treatment initiation were related to reimbursement, insurance coverage, or out-of-pocket costs. In biologic-naïve patients, the most frequent reasons for infliximab-dyyb treatment initiation were targeted therapy (64.1%), improved efficacy (15.4%), and new drug availability (12.8%).
Thirty-three patients had dose escalation at some point during the follow-up [16 (41%) in biologic naïve, 12 (21%) in patients switching from RP infliximab, and 5 (26%) in patients switching from other biologics]. A total of 13 patients had dose reductions at any time during the follow-up period [9 (23%) in biologic naïve, 3 (5%) in patients switching from RP infliximab, and 1 (5%) in patients switching from other biologics.]
Thirty-one patients in total (27%) did not reach 12 months’ follow-up. Six patients discontinued due to an AE, 1 discontinued due to lack of efficacy, 17 were lost to follow-up, 3 patients chose to withdraw, and 4 patients discontinued for other reasons. The 6 AEs that caused study withdrawal were 1 development of antidrug antibodies, 1 case of community-acquired pneumonia, 1 hypersensitivity reaction, 1 liver abscess, 1 case of drug-induced lupus, and 1 case of psoriasiform dermatitis and joint pain.
Clinical Results
Descriptive results for clinical outcomes are presented in Table 2. Figures depicting remission and response results over time are presented in the Supplementary Material. In CD patients during the follow-up period, HBI scores did not change significantly. Mean (SD) HBI scores were 3.45 (3.04) at baseline, 3.11 (3.27) at 3 months’ follow-up, and 2.98 (2.61) at 12 months’ follow-up (p = 0.3988).
In UC patients, the mean (SD) baseline pMAYO score was 3.85 (3.05). The pMAYO score improved significantly, to 1.44 (1.94) at 3 months’ follow-up and 0.90 (1.47) at 12 months’ follow-up (p < 0.0001). Among UC patients who were biologic-naïve, pMAYO scores improved significantly over the course of the intervention, from a mean (SD) of 5.67 (2.25) at baseline to 1.41 (1.42) at 3 months’ follow-up and 1.09 (1.22) at 12 months’ follow-up (p < 0.0001). Among the subgroup of UC patients switched from RP infliximab, the pMAYO score did not change significantly over the course of the intervention, from a mean (SD) of 1.38 (1.83) at baseline to 0.56 (1.20) at 3 months’ follow-up and to 0.29 (0.85) at 12 months’ follow-up (p = 0.0103).
At baseline, 35.4% of enrolled UC patients were classified as in remission (Table 2). At 12 months’ follow-up, 87.1% of enrolled UC patients were classified as in remission (p < 0.0001). In UC patients who were biologic naïve, 5.6% were classified as in remission at baseline. This proportion increased significantly at 12 months’ follow-up to 90.9% (p = 0.0015). UC patients switched from RP infliximab indicated an improving trend for rate of remission from 71.4% at baseline to 94.1% at 12 months’ follow-up; however, it was not statistically significant (p = 0.1007).
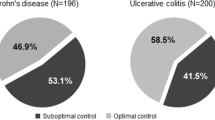
Of the enrolled CD patients, 72.7% were classified as in remission at baseline (Table 2). After 12 months of infliximab-dyyb treatment, 77.1% of CD patients were classified as in remission (p = 0.8011). In CD patients switched from RP infliximab, the remission rate was maintained over the duration of the study from a baseline rate of 77.8% to a 12-month remission rate of 76.7% (p = 0.1077). In CD patients, 30.8% of biologic-naïve users and 6.7% of those who switched from RP infliximab demonstrated a clinical response to treatment.
Patient-Reported Outcomes
PROs improved significantly from baseline to 12 months’ follow-up in nearly all questionnaires administered to participants. Descriptive results at each visit are presented in Table 3. Results for changes from baseline from the MMRM model are depicted in Figs. 2 and 3. Higher scores reflect better quality of life in all the instruments except the WPAI, GAD-7, and PHQ-8, where lower scores reflect better quality of life. SIBDQ, EQ-VAS, all domains of WPAI, the effectiveness domain of TSQM, GAD-7, and PHQ-8 scores significantly improved from baseline to 12 months’ follow-up. Significant improvements were observed in SIBDQ scores from baseline to 12 months in each cohort (all p < 0.05). The SIBDQ score for the IBD cohort increased by 7 points at 3 months and by 9 points at the 12-month visit. Biologic-naïve users showed an improvement of 16 points, whereas patients who switched from RP infliximab showed an increase of 3 points (Fig. 2). EQ-VAS scores improved in the cohort of all enrolled patients compared to baseline (9-point improvement; p < 0.001), in biologic-naïve users (14-point improvement; p = 0.002), and in switchers from RP infliximab (5-point improvement; p = 0.010) (Fig. 2).
Changes from baseline in daily impairment (WPAI), effectiveness (TSQM), PHQ-8, GAD-7. *Denotes statistically significant (p < 0.05) change from mixed model for repeated measures (MMRM). IBD inflammatory bowel disease, SIBDQ Short Inflammatory Bowel Disease Questionnaire, EQ-VAS EuroQol Visual Analogue Scale
IBD-related impairment in daily activities, measured by the WPAI, decreased significantly in the cohort of all patients and in biologic-naïve users (all p < 0.05) (Table 3). Results from MMRM analysis indicated that the WPAI score decreased by 12 points at the 3-month visit and by 20 points at the 12-month visit for the IBD cohort. Biologic-naïve users showed a decrease of 25 points at the 3-month visit and 36 points at the 12-month visit. Switchers from RP infliximab had a decrease of 8 points by 12 months (Fig. 3). Patient-perceived treatment effectiveness, measured by the TSQM, also improved significantly in the cohort of all patients and in biologic-naïve users (both p < 0.001) (Table 3). Results from the MMRM model indicated that patient-perceived treatment effectiveness increased by 6 points at 3 months and 13 points at 12 months for the IBD cohort. Biologic-naïve users showed an increase of 17 points at 3 months and 26 points at 12 months. Switchers from RP infliximab had an increase of 2 points (Fig. 3). The PHQ-8 and GAD-7 scores decreased over time for the IBD cohort and for biologic-naïve patients, indicating improved quality of life (Table 3). Results from an MMRM analysis indicated that, for the IBD cohort, PHQ-8 and GAD-7 scores decreased by 3.15 and 2.65 at 12 months, respectively. Biologic-naïve users showed a decrease of 4.54 points and 2.65 points for the PHQ-8 and GAD-7 at 12 months, respectively. Switchers from RP infliximab had a decrease of 1.21 for the PHQ-8 and 0.39 for the GAD-7 at 12 months (Fig. 3).
Healthcare Resource Use
An IBD-related hospitalization was recorded in 9.6% of patients within the baseline period and in 1.2% of patients within the 12-month observation period (Table 4). An ED visit was recorded in 10.4% of patients within the baseline period and in 3.6% within the 12-month observation period. The mean (SD) number of non-infusion gastroenterologist visits was 0.78 (1.67) visits per patient during the baseline period and 0.69 (0.78) visits per patient at 12 months.
Adverse Events
Fifty-nine AEs were reported in 40 (40/115; 34.8%) patients. Of these, 29 (49.2%) were mild, 23 (39.0%) were moderate, and 7 (11.9%) were severe. Twenty-two AEs occurred that were classified by the enrolling physician as related to study treatment. The most frequently reported AEs related to study treatment were: gastrointestinal disorders (n = 8; 6.95%); infusion-related reactions (n = 4; 3.5%); platelet, bleeding, and clotting disorders (n = 2; 1.72%); and hypersensitivity reactions (n = 2; 1.72%). Of severe AEs that occurred during the study period, all but one were deemed by the enrolling physician to be unrelated to the intervention. The severe AE related to infliximab-dyyb treatment was a severe hypersensitivity reaction. Overall, AEs occurred at rates consistent with the known AE profile for infliximab.
Discussion
This study assessed the effectiveness of infliximab-dyyb among IBD patients in terms of clinical outcomes, quality of life, work productivity, and resource utilization. These outcomes were examined among patients who switched from RP infliximab or other biologics and biologic-naïve patients initiating infliximab. Overall, this study showed positive response and remission outcomes in biologic-naïve IBD patients. Clinical response was observed in 72.7% of UC and 30.8% of CD biologic-naïve users initiating infliximab-dyyb treatment. However, 65.0% of CD biologic-naïve users were already in remission at baseline (scoring < 5 on HBI), which limited their room for clinical response (defined as an HBI improvement of ≥ 3 points). At the end of 12 months’ follow-up, 90.9% of UC and 84.6% of CD biologic-naïve users were in remission. These rates are consistent with other studies of infliximab-dyyb as a patient’s first biologic therapy for IBD. A 2017 meta-analysis by Komaki et al. reviewed 11 observational studies of patients with active CD or UC treated with CT-P13 (switched from RP infliximab or biologic naïve) [19]. Consistent with our findings, pooled clinical response rates at 24–30 weeks were 77% in UC and 77% in CD, and pooled clinical remission rates were 42% in UC and 60% in CD. Several other studies have suggested that infliximab-dyyb is effective and safe in biologic-naïve patients [20,21,22,23,24]. A large comparative study of infliximab-naïve patients with IBD initiating either RP infliximab or CT-P13 concluded that infliximab-dyyb was equally efficacious, with no clinically meaningful differences [25].
Our findings on clinical outcomes among patients switching from RP infliximab to infliximab-dyyb are consistent with those of prior studies [13, 16, 22, 26,27,28,29,30,31,32,33,34,35,36,37,38,39]. In the present study, at 12 months’ follow-up, clinical remission was observed in 94.1% of UC and 76.7% of CD patients switched from RP infliximab to infliximab-dyyb. Jung et al. studied 59 IBD patients switching from RP infliximab to CT-P13, and observed that 92.6% of CD patients and 66.7% of UC patients maintained similar efficacy compared with infliximab [13]. Smits et al. [16] studied 83 RP infliximab–treated IBD patients who switched to CT-P13, and similarly found that over 80% of those patients maintained clinical remission. Furthermore, Smits et al. found that IBD activity remained stable after switching. In another published study, Chaparro et al. found unfavorable results in users who switched from RP infliximab to CT-P13 [40]. However, Chaparro et al. explained that the higher risk of clinical relapse observed in patients switched to CT-P13 was not supported by objective markers of inflammation and may have been due to the nocebo effect.
PROs showed significant improvements over time for all IBD patients initiating infliximab-dyyb (n = 115). Scores on the SIBDQ, the EQ-VAS, all domains of the WPAI, the effectiveness domain of the TSQM, the GAD-7, and the PHQ-8 significantly improved from baseline to 12 months’ follow-up. In biologic-naïve users, the current study observed a 16-point improvement in SIBDQ score from baseline to 12 months’ follow-up. In a randomized phase 3 non-inferiority study, Ye et al. [39] in 2019 observed an 18.6- and a 16.7-point improvement in the 30-week SIBDQ score in biologic-naïve CD patients initiating RP infliximab or CT-P13, respectively. In the present study, biologic-naïve patients initiating infliximab-dyyb also demonstrated significant improvements in scores on the EQ-VAS; the presenteeism, overall work impairment, and daily activity impairment domains of the WPAI; the effectiveness and side effects domains of the TSQM; the GAD-7; and the PHQ-8.
PROs were maintained in users switched from RP infliximab to infliximab-dyyb. Our findings using PROs are novel and valuable, in that there have been few observational studies that have assessed PROs, specifically work productivity, psychological outcomes, and treatment satisfaction in IBD. In this study, SIBDQ, EQ-VAS, TSQM, GAD-7, and PHQ-8 measures were maintained from baseline to 12-month follow-up in the cohort of patients switching from RP infliximab to infliximab-dyyb. WPAI scores improved significantly in this cohort in the domains of absenteeism and overall work impairment. Our findings are consistent with the randomized, non-inferiority, double-blind NOR-SWITCH study, which assigned patients on stable RP infliximab treatment in a 1:1 ratio to either continue treatment with RP infliximab or be switched to CT-P13, observed that improvements in SF-36, EQ-5D, and WPAI scores were not statistically different between RP infliximab and infliximab-dyyb users [32].
To our knowledge, this is the first study to prospectively evaluate treatment satisfaction in patients switched from RP infliximab to infliximab-dyyb for IBD. Our findings, based on TSQM, suggest that these patients remained satisfied with infliximab-dyyb in terms of its effectiveness, side effects, and convenience.
Our findings as to resource utilization suggest that hospitalization, ED visits, and outpatient visits decreased over time, which could be due to improvements observed in patients’ clinical outcomes and PROs. Regarding AEs, no new safety signals were observed during the conduct of this study. Although the number of patients in this study is small and direct comparisons cannot be made, the AE rates observed in this study are in line with what has previously been reported with RP infliximab [41,42,43,44].
This study has several limitations to consider. The study did not collect data on therapeutic drug monitoring. Therefore, any correlations between drug concentrations and outcomes were not examined. The study had a sample size smaller than anticipated. This was likely due in part to the prospective observational nature of the study. One of the main difficulties in patient recruitment was the lack of formulary availability/insurance coverage of infliximab-dyyb during patient enrollment at the time of study initiation. Due to the lack of uptake of infliximab-dyyb at the time of patient enrolment in the US, fewer study sites than planned were able to identify and recruit patients. This was largely because infliximab-dyyb was not on their formulary or because patient insurance would not cover infliximab-dyyb. Additionally, some patients were lost to follow-up. However, nearly 70% of patients completed all four visits. Due to the low sample size for patient subgroups, we did not control for covariates in our MMRM models. However, demographic, and clinical characteristics in our study were not time varying; therefore, we do not anticipate any change in the directionality of our conclusions. Further, due to the overall low sample size (n < 20) and multiple patient groups, results from the subgroup of patients switching from other biologics should be interpreted with caution. Future studies with larger sample sizes may confirm the findings from this study.
Conclusions
In this prospective, observational study, we evaluated real-world clinical outcomes, PROs, and healthcare resource use associated with infliximab-dyyb for IBD among biologic-naïve patients and patients switching from RP infliximab or other biologics. For biologic-naïve UC patients, clinical outcomes improved significantly, while for biologic-naïve CD patients, they were maintained. Consistent with findings across studies of other immunological diseases, our study found that patients who switched from RP infliximab to infliximab-dyyb maintained clinical outcomes and remission status. The patient-reported quality-of-life and work productivity outcomes improved among biologic-naïve patients and were maintained for patients switched from RP infliximab. AEs occurred at a rate consistent with the known AE profile of RP infliximab, but it must be acknowledged that the number of patients in this study is small and direct comparisons with other studies should be made with caution. To our knowledge, this is the first prospective study of real-world outcomes in IBD patients treated with infliximab-dyyb in North America. The results of this study provide valuable data concerning the use of infliximab-dyyb in clinical practice for patients with IBD.
References
Hazel K, O’Connor A. Emerging treatments for inflammatory bowel disease. Ther Adv Chronic Dis. 2020;11:2040622319899297.
Hanauer SB, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–9.
Rutgeerts P, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–76.
Sands BE, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350(9):876–85.
Sands BE, et al. Long-term treatment of rectovaginal fistulas in Crohn’s disease: response to infliximab in the ACCENT II study. Clin Gastroenterol Hepatol. 2004;2(10):912–20.
Veloz MFG, et al. Long-term follow up after switching from original infliximab to an infliximab biosimilar: real-world data. Ther Adv Gastroenterol. 2019;12:1756284819858052–1756284819858052.
Kapoor S, et al. Real-life tolerability and effectiveness of adalimumab biosimilar in ankylosing spondylitis: the adalimumab biosimilar patient registry data. ACR Open Rheumatol. 2019;1(8):480–4.
FDA approves Inflectra, a biosimilar to Remicade. 2016.https://www.drugs.com/newdrugs/fda-approves-inflectra-infliximab-dyyb-biosimilar-remicade-4364.html.
Martín-Gutiérrez N, Sánchez-Hernández JG, Rebollo N, Pordomingo AF, Muñoz F, Otero MJ. Long-term effectiveness and pharmacokinetics of the infliximab biosimilar CT-P13 after switching from the originator during the treatment of inflammatory bowel disease [published online ahead of print, 2020 Oct 28]. Eur J Hosp Pharm. 2020. https://doi.org/10.1136/ejhpharm-2020-002410.
Sagami S, et al. Post-marketing analysis for biosimilar CT-P13 in inflammatory bowel disease compared with external data of originator infliximab in Japan. J Gastroenterol Hepatol. 2021;36(8):2091–100.
Farkas K, et al. Efficacy of the new infliximab biosimilar CT-P13 induction therapy in Crohn’s disease and ulcerative colitis—experiences from a single center. Expert Opin Biol Ther. 2015;15(9):1257–62.
Gecse KB, et al. Efficacy and safety of the biosimilar infliximab CT-P13 treatment in inflammatory bowel diseases: a prospective, multicentre, Nationwide Cohort. J Crohns Colitis. 2016;10(2):133–40.
Jung YS, et al. Efficacy and safety of CT-P13, a biosimilar of infliximab, in patients with inflammatory bowel disease: a retrospective multicenter study. J Gastroenterol Hepatol. 2015;30(12):1705–12.
Kang YS, et al. Clinical experience of the use of CT-P13, a biosimilar to infliximab in patients with inflammatory bowel disease: a case series. Dig Dis Sci. 2015;60(4):951–6.
Nikiphorou E, et al. Clinical effectiveness of CT-P13 (Infliximab biosimilar) used as a switch from Remicade (infliximab) in patients with established rheumatic disease. Report of clinical experience based on prospective observational data. Expert Opin Biol Ther. 2015;15(12):1677–83.
Smits LJ, et al. Clinical outcomes following a switch from Remicade® to the biosimilar CT-P13 in inflammatory bowel disease patients: a prospective observational cohort study. J Crohns Colitis. 2016;10(11):1287–93.
Tanaka Y, et al. Safety and efficacy of CT-P13 in Japanese patients with rheumatoid arthritis in an extension phase or after switching from infliximab. Mod Rheumatol. 2017;27(2):237–45.
Desai RJ, Kim SC, Curtis JR, et al. Methodologic considerations for noninterventional studies of switching from reference biologic to biosimilars. Pharmacoepidemiol Drug Saf. 2020;29(7):757–769.https://doi.org/10.1002/pds.4809.
Komaki Y, et al. Systematic review with meta-analysis: the efficacy and safety of CT-P13, a biosimilar of anti-tumour necrosis factor-α agent (infliximab), in inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;45(8):1043–57.
Ratnakumaran R, et al. Efficacy and tolerability of initiating, or switching to, infliximab biosimilar CT-P13 in inflammatory bowel disease (IBD): a large single-centre experience. Scand J Gastroenterol. 2018;53(6):700–7.
Kaniewska M, Moniuszko A, Rydzewska G. The efficacy and safety of the biosimilar product (Inflectra(®)) compared to the reference drug (Remicade(®)) in rescue therapy in adult patients with ulcerative colitis. Prz Gastroenterol. 2017;12(3):169–74.
Høivik ML, et al. Switching from originator to biosimilar infliximab—real world data of a prospective 18 months follow-up of a single-centre IBD population. Scand J Gastroenterol. 2018;53(6):692–9.
Veloz MFG, et al. Switching from reference infliximab to CT-P13 in patients with inflammatory bowel disease: results of a multicenter study after 12 months. Rev Esp Enferm Dig. 2018;110(9):564–70.
Armuzzi A, et al. The PROSIT cohort of infliximab biosimilar in IBD: a prolonged follow-up on the effectiveness and safety across Italy. Inflamm Bowel Dis. 2019;25(3):568–79.
Meyer A, et al. Effectiveness and safety of reference infliximab and biosimilar in Crohn disease: A French Equivalence Study. Ann Intern Med. 2019;170(2):99–107.
Bronswijk M, et al. Evaluating efficacy, safety, and pharmacokinetics after switching from infliximab originator to biosimilar CT-P13: experience from a large tertiary referral center. Inflamm Bowel Dis. 2020;26(4):628–34.
Ebada MA, et al. An updated systematic review and meta-analysis about the safety and efficacy of infliximab biosimilar, CT-P13, for patients with inflammatory bowel disease. Int J Colorectal Dis. 2019;34(10):1633–52.
Gheorghe C, Svoboda P, Mateescu B. Effectiveness and safety of biosimilar infliximab (CT-P13) in a real-life setting in patients with Crohn’s disease or ulcerative colitis. J Drug Assess. 2019;8(1):129–34.
Guerra Veloz MF, et al. Loss of efficacy and safety of the switch from infliximab original to infliximab biosimilar (CT-P13) in patients with inflammatory bowel disease. World J Gastroenterol. 2018;24(46):5288–96.
Ho SL, et al. Effectiveness of switching from reference product Infliximab to Infliximab-Dyyb in patients with inflammatory bowel disease in an integrated healthcare system in the United States: a retrospective, propensity score-matched, non-inferiority cohort study. BioDrugs. 2020;34(3):395–404.
Ilias A, et al. Outcomes of patients with inflammatory bowel diseases switched from maintenance therapy with a biosimilar to remicade. Clin Gastroenterol Hepatol. 2019;17(12):2506-2513.e2.
Jørgensen KK, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017;389(10086):2304–16.
Kim NH, et al. Long-term efficacy and safety of CT-P13, a biosimilar of infliximab, in patients with inflammatory bowel disease: a retrospective multicenter study. J Gastroenterol Hepatol. 2019;34(9):1523–32.
Nakagawa T, et al. Infliximab biosimilar CT-P13 is interchangeable with its originator for patients with inflammatory bowel disease in real world practice. Intest Res. 2019;17(4):504–15.
Petitdidier N, et al. Patients’ perspectives after switching from infliximab to biosimilar CT-P13 in patients with inflammatory bowel disease: a 12-month prospective cohort study. Dig Liver Dis. 2019;51(12):1652–60.
Plevris N, et al. Implementation of CT-P13 via a managed switch programme in Crohn’s disease: 12-month real-world outcomes. Dig Dis Sci. 2019;64(6):1660–7.
Strik AS, et al. Serum concentrations after switching from originator infliximab to the biosimilar CT-P13 in patients with quiescent inflammatory bowel disease (SECURE): an open-label, multicentre, phase 4 non-inferiority trial. Lancet Gastroenterol Hepatol. 2018;3(6):404–12.
van Hoeve K, et al. Efficacy, pharmacokinetics, and immunogenicity is not affected by switching from infliximab originator to a biosimilar in pediatric patients with inflammatory bowel disease. Ther Drug Monit. 2019;41(3):317–24.
Ye BD, et al. Efficacy and safety of biosimilar CT-P13 compared with originator infliximab in patients with active Crohn’s disease: an international, randomised, double-blind, phase 3 non-inferiority study. Lancet. 2019;393(10182):1699–707.
Chaparro M, et al. Effectiveness and safety of the switch from Remicade® to CT-P13 in patients with inflammatory bowel disease. J Crohns Colitis. 2019;13(11):1380–6.
Reinisch W, et al. Long-term infliximab maintenance therapy for ulcerative colitis: the ACT-1 and-2 extension studies. Inflamm Bowel Dis. 2012;18(2):201–11.
Sandborn WJ, et al. One-year maintenance outcomes among patients with moderately-to-severely active ulcerative colitis who responded to induction therapy with adalimumab: subgroup analyses from ULTRA 2. Aliment Pharmacol Ther. 2013;37(2):204–13.
Vermeire S, et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut. 2007;56(9):1226–31.
Yoshida K, et al. Scheduled infliximab monotherapy to prevent recurrence of Crohn’s disease following ileocolic or ileal resection: a 3-year prospective randomized open trial. Inflamm Bowel Dis. 2012;18(9):1617–23.
Acknowledgements
Funding
Research funding was provided by Pfizer. Pfizer also funded the journal’s Rapid Service and Open Access Fees.
Medical Writing, Editorial, and Other Assistance
Editorial assistance was provided by Gregory Poorman of OPEN Health and was funded by Pfizer. Nehemiah Kebede assisted with the data analysis. The authors would also like to acknowledge Patrick Edmundson for data collection and site management, Chris Atzinger for conceptualization and study management, and Seema Haider and John Kelton for their contributions in conceptualization and study design. Gregory Poorman, Nehemiah Kebede, and Patrick Edmundson are employees of OPEN Health. Chris Atzinger is a former employee of OPEN Health. John Kelton is an employee of Pfizer. Seema Haider is a former employee of Pfizer.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
Bincy Abraham, Arif Soonasra, Jennifer Stephens, Hrishikesh Kale, and Dipen Patel contributed to study design and conception. Bertus Eksteen and Khan Need contributed to data collection. Hrishikesh Kale and Jennifer Stephens contributed to the data analysis. All authors contributed to manuscript writing and editing. All authors reviewed and approved the final manuscript.
Disclosures
Dr. Bincy Abraham received personal fees from Pfizer, Janssen, AbbVie, Takeda, Samsung Bioepis, Ferring, and Bristol Myers Squibb outside the submitted work. Dr. Bertus Eksteen has nothing to disclose. Dr. Khan Nedd has nothing to disclose. Hrishikesh Kale, Dipen Patel, and Jennifer Stephens are employees of OPEN Health and were paid consultants to Pfizer in connection with the development of this manuscript. Dipen Patel and Jennifer Stephens own OPEN Health stock. Ahmed Shelbaya, Richard Chambers, and Arif Soonasra are employees of Pfizer and own Pfizer stock. Ahmed Shelbaya is also an employee of the Mailman School of Public Health at Columbia University.
Compliance with Ethics Guidelines
The final protocol, any amendments, and informed consent documentation were reviewed and approved by a local data protection agency for each site participating in the study. Informed consent was obtained from all participants included in the study. Full details of institutional review boards that approved the study, along with relevant reference numbers can be found in the Supplementary Material.
Data Availability
Upon request and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual anonymized participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Abraham, B., Eksteen, B., Nedd, K. et al. Impact of Infliximab-dyyb (Infliximab Biosimilar) on Clinical and Patient-Reported Outcomes: 1-Year Follow-up Results from an Observational Real-World Study Among Patients with Inflammatory Bowel Disease in the US and Canada (the ONWARD Study). Adv Ther 39, 2109–2127 (2022). https://doi.org/10.1007/s12325-022-02104-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02104-6