Abstract
Introduction
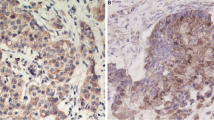
Ovarian cancer has a high mortality rate due to difficulties in early detection and chemotherapy resistance. Human epididymal protein 4 (HE4) has been adopted as a novel serum biomarker for early ovarian cancer diagnosis, and the presence of Lewis y antigen modifications on HE4 in ovarian cancer cell lines has been detected in previous studies. The aim of this study was to analyze the expression of HE4 and Lewis y antigen in human ovarian cancer in order to find a correlation between them, as well as with the clinical pathological parameters of patients with ovarian cancer.
Methods
Immunohistochemistry was used to detect the respective expression of these compounds in two patient groups (chemotherapy-resistant and chemotherapy-sensitive) containing a total of 95 patients. Then, a bioinformatic approach was adopted and online large sample databases (TCGA, CCLE, and GTEx; Metascape, Cytoscape) were used to explore the potential mechanisms of action of these compounds.
Results
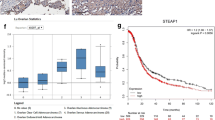
The results of this study demonstrate that high HE4 and Lewis y expression could be used as markers for chemotherapy resistance and poor prognosis in patients with ovarian cancer. These two expression events were widely correlated in various cancer tissues and are thought to act by activating the p38 mitogen-activated protein kinases (MAPK) pathway and inducing Vascular Endothelial Growth Factor A (VEGFA), Prostaglandin-Endoperoxide Synthase 2 (PTGS2), Early Growth Response 1 (EGR1), and Hypoxia-Inducible Factor 1-Alpha (HIFI1A), thereby promoting malignant biological behavior and resistance in ovarian cancer.
Conclusions
These findings not only reveal the possible mechanism by which HE4 and Lewis y antigen affect ovarian cancer but also identify a four-gene signature that could be very useful in ovarian cancer detection and/or the development of new targeted therapies.




Similar content being viewed by others
References
Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3–14.
Hellstrom I, Raycraft J, Hayden-Ledbetter M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63(13):3695–700.
Drapkin R, von Horsten HH, Lin Y, et al. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005;65(6):2162–9.
Scaletta G, Plotti F, Luvero D, et al. The role of novel biomarker HE4 in the diagnosis, prognosis and follow-up of ovarian cancer: a systematic review. Expert Rev Anticancer Ther. 2017;17(9):827–39.
Zhang L, Chen Y, Wang K. Comparison of CA125, HE4, and ROMA index for ovarian cancer diagnosis. Curr Probl Cancer. 2019;43(2):135–44.
Dochez V, Caillon H, Vaucel E, Dimet J, Winer N, Ducarme G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res. 2019;12(1):28.
Kitamura K, Stockert E, Garin-Chesa P, et al. Specificity analysis of blood group Lewis-y (Le(y)) antibodies generated against synthetic and natural Le(y) determinants. Proc Natl Acad Sci U S A. 1994;91(26):12957–61.
Hokke CH, Neeleman AP, Koeleman CA, van den Eijnden DH. Identification of an α3-fucosyltransferase and a novel α2-fucosyltransferase activity in cercariae of the schistosome Trichobilharzia ocellata: biosynthesis of the Fucα1→2Fucα1→3[Gal(NAc)β1→4]GlcNAc sequence. Glycobiology. 1998;8(4):393–406.
Dettke M, Palfi G, Loibner H. Activation-dependent expression of the blood group-related Jewis Y antigen on peripheral blood granulocytes. J Leukoc Biol. 2000;68(4):511–4.
Yan LM, Lin B, Zhu LC, et al. Enhancement of the adhesive and spreading potentials of ovarian carcinoma RMG-1 cells due to increased expression of integrin alpha5beta1 with the Lewis Y-structure on transfection of the alpha1,2-fucosyltransferase gene. Biochimie. 2010;92(7):852–7.
Li F, Lin B, Hao Y, et al. Lewis Y promotes growth and adhesion of ovarian carcinoma-derived RMG-I cells by upregulating growth factors. Int J Mol Sci. 2010;11(10):3748–59.
Liu J, Lin B, Hao Y, et al. Lewis y antigen promotes the proliferation of ovarian carcinoma-derived RMG-I cells through the PI3K/Akt signaling pathway. J Exp Clin Cancer Res. 2009;28:154.
Zhuang H, Gao J, Hu Z, Liu J, Liu D, Lin B. Co-expression of Lewis y antigen with human epididymis protein 4 in ovarian epithelial carcinoma. PLoS ONE. 2013;8(7):e68994.
NCCN Clinical Practice Guidelines in Oncology-Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer (Version 3.2021). .http://www.nccn.org. Accessed 9 Sept 2021
Zhu LC, Gao J, Hu ZH, et al. Membranous expressions of Lewis y and CAM-DR-related markers are independent factors of chemotherapy resistance and poor prognosis in epithelial ovarian cancer. Am J Cancer Res. 2015;5(2):830–43.
Sinicrope FA, Ruan SB, Cleary KR, Stephens LC, Lee JJ, Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995;55(2):237–41.
Lu CD, Altieri DC, Tanigawa N. Expression of a novel antiapoptosis gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res. 1998;58(9):1808–12.
Carithers LJ, Ardlie K, Barcus M, et al. A novel approach to high-quality postmortem tissue procurement: the GTEx project. Biopreserv Biobank. 2015;13(5):311–9.
Barretina J, Caponigro G, Stransky N, et al. Addendum: the cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2019;565(7738):E5–6.
Gao S, Zhu L, Feng H, et al. Gene expression profile analysis in response to alpha1,2-fucosyl transferase (FUT1) gene transfection in epithelial ovarian carcinoma cells. Tumour Biol. 2016;37(9):12251–62.
Zhu L, Guo Q, Jin S, et al. Analysis of the gene expression profile in response to human epididymis protein 4 in epithelial ovarian cancer cells. Oncol Rep. 2016;36(3):1592–604.
Zhu L, Zhuang H, Wang H, et al. Overexpression of HE4 (human epididymis protein 4) enhances proliferation, invasion and metastasis of ovarian cancer. Oncotarget. 2016;7(1):729–44.
Zhou Y, Zhou B, Pache L, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523.
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8(Suppl 4):S11.
Wang H, Zhu L, Gao J, Hu Z, Lin B. Promotive role of recombinant HE4 protein in proliferation and carboplatin resistance in ovarian cancer cells. Oncol Rep. 2015;33(1):403–12.
Zhuang H, Tan M, Liu J, et al. Human epididymis protein 4 in association with Annexin II promotes invasion and metastasis of ovarian cancer cells. Mol Cancer. 2014;13:243.
Wang J, Deng L, Zhuang H, et al. Interaction of HE4 and ANXA2 exists in various malignant cells-HE4-ANXA2-MMP2 protein complex promotes cell migration. Cancer Cell Int. 2019;19:161.
Wang A, Jin C, Tian X, Wang Y, Li H. Knockdown of HE4 suppresses aggressive cell growth and malignant progression of ovarian cancer by inhibiting the JAK/STAT3 pathway. Biol Open. 2019; 8(9):bio043570.
Liu D, Kong D, Li J, et al. HE4 level in ascites may assess the ovarian cancer chemotherapeutic effect. J Ovarian Res. 2018;11(1):47.
Lee S, Choi S, Lee Y, Chung D, Hong S, Park N. Role of human epididymis protein 4 in chemoresistance and prognosis of epithelial ovarian cancer. J Obstet Gynaecol Res. 2017;43(1):220–7.
Aarenstrup Karlsen M, Hogdall C, Nedergaard L, et al. HE4 as a predictor of adjuvant chemotherapy resistance and survival in patients with epithelial ovarian cancer. APMIS. 2016;124(12):1038–45.
Moore RG, Hill EK, Horan T, et al. HE4 (WFDC2) gene overexpression promotes ovarian tumor growth. Sci Rep. 2014;4:3574.
Nonaka M, Ma BY, Murai R, et al. Glycosylation-dependent interactions of C-type lectin DC-SIGN with colorectal tumor-associated Lewis glycans impair the function and differentiation of monocyte-derived dendritic cells. J Immunol. 2008;180(5):3347–56.
Tan M, Zhu L, Zhuang H, et al. Lewis Y antigen modified CD47 is an independent risk factor for poor prognosis and promotes early ovarian cancer metastasis. Am J Cancer Res. 2015;5(9):2777–87.
Gao J, Hu Z, Liu J, et al. Expression of CD147 and Lewis y antigen in ovarian cancer and their relationship to drug resistance. Med Oncol. 2014;31(5):920.
Zhuang H, Tan M, Liu J, et al. The expression of annexin II and Lewis y antigen in ovarian epithelial tumors and the correlation between them. Tumour Biol. 2015;36(4):2343–9.
Cai M, Jin S, Deng L, et al. Lewis y antigen promotes p27 degradation by regulating ubiquitin-proteasome activity. Oncotarget. 2017;8(66):110064–76.
Burotto M, Chiou VL, Lee JM, Kohn EC. The MAPK pathway across different malignancies: a new perspective. Cancer. 2014;120(22):3446–56.
Ribeiro JR, Schorl C, Yano N, et al. HE4 promotes collateral resistance to cisplatin and paclitaxel in ovarian cancer cells. J Ovarian Res. 2016;9(1):28.
Hao Y, Zhu L, Yan L, et al. c-Fos mediates alpha1, 2-fucosyltransferase 1 and Lewis y expression in response to TGF-beta1 in ovarian cancer. Oncol Rep. 2017;38(6):3355–66.
Shan X, Aziz F, Tian LL, Wang XQ, Yan Q, Liu JW. Ginsenoside Rg3-induced EGFR/MAPK pathway deactivation inhibits melanoma cell proliferation by decreasing FUT4/LeY expression. Int J Oncol. 2015;46(4):1667–76.
Hybel TE, Dietrichs D, Sahana J, et al. Simulated microgravity influences VEGF, MAPK, and PAM signaling in prostate cancer cells. Int J Mol Sci. 2020;21(4):1263.
Wang S, Xiao Z, Hong Z, et al. FOXF1 promotes angiogenesis and accelerates bevacizumab resistance in colorectal cancer by transcriptionally activating VEGFA. Cancer Lett. 2018;439:78–90.
Guo J, Chen M, Ai G, Mao W, Li H, Zhou J. Hsa_circ_0023404 enhances cervical cancer metastasis and chemoresistance through VEGFA and autophagy signaling by sponging miR-5047. Biomed Pharmacother. 2019;115:108957.
Cairns J, Ingle JN, Kalari KR, et al. The lncRNA MIR2052HG regulates ERalpha levels and aromatase inhibitor resistance through LMTK3 by recruiting EGR1. Breast Cancer Res. 2019;21(1):47.
Sun M, Nie FQ, Zang C, et al. The pseudogene DUXAP8 promotes non-small-cell lung cancer cell proliferation and invasion by epigenetically silencing EGR1 and RHOB. Mol Ther. 2017;25(3):739–51.
Stamatakis K, Jimenez-Martinez M, Jimenez-Segovia A, et al. Prostaglandins induce early growth response 1 transcription factor mediated microsomal prostaglandin E2 synthase up-regulation for colorectal cancer progression. Oncotarget. 2015;6(37):39941–59.
Parra E, Gutierrez L, Ferreira J. Association of increased levels of TGF-beta1 and p14ARF in prostate carcinoma cell lines overexpressing Egr-1. Oncol Rep. 2014;32(5):2191–8.
Shajahan-Haq AN, Boca SM, Jin L, et al. EGR1 regulates cellular metabolism and survival in endocrine resistant breast cancer. Oncotarget. 2017;8(57):96865–84.
Tang T, Zhu Q, Li X, et al. Protease Nexin I is a feedback regulator of EGF/PKC/MAPK/EGR1 signaling in breast cancer cells metastasis and stemness. Cell Death Dis. 2019;10(9):649.
Wu Y, Li D, Wang Y, et al. Beta-defensin 2 and 3 promote bacterial clearance of Pseudomonas aeruginosa by inhibiting macrophage autophagy through downregulation of early growth response gene-1 and c-FOS. Front Immunol. 2018;9:211.
Parmakhtiar B, Burger RA, Kim JH, Fruehauf JP. HIF inactivation of p53 in ovarian cancer can be reversed by topotecan, restoring cisplatin and paclitaxel sensitivity. Mol Cancer Res. 2019;17(8):1675–86.
Zhang X, Qi Z, Yin H, Yang G. Interaction between p53 and Ras signaling controls cisplatin resistance via HDAC4- and HIF-1alpha-mediated regulation of apoptosis and autophagy. Theranostics. 2019;9(4):1096–114.
Zhang W, Yuan W, Song J, Wang S, Gu X. LncRNA CPS1-IT1 suppresses EMT and metastasis of colorectal cancer by inhibiting hypoxia-induced autophagy through inactivation of HIF-1alpha. Biochimie. 2018;144:21–7.
Ko CJ, Lan SW, Lu YC, et al. Inhibition of cyclooxygenase-2-mediated matriptase activation contributes to the suppression of prostate cancer cell motility and metastasis. Oncogene. 2017;36(32):4597–609.
Xu H, Lin F, Wang Z, et al. CXCR2 promotes breast cancer metastasis and chemoresistance via suppression of AKT1 and activation of COX2. Cancer Lett. 2018;412:69–80.
Ooki A, Del Carmen Rodriguez Pena M, Marchionni L, et al. YAP1 and COX2 coordinately regulate urothelial cancer stem-like cells. Cancer Res. 2018;78(1):168–81.
Yu JL, Gao X. MicroRNA 1301 inhibits cisplatin resistance in human ovarian cancer cells by regulating EMT and autophagy. Eur Rev Med Pharmacol Sci. 2020;24(4):1688–96.
Liang F, Ren C, Wang J, et al. The crosstalk between STAT3 and p53/RAS signaling controls cancer cell metastasis and cisplatin resistance via the Slug/MAPK/PI3K/AKT-mediated regulation of EMT and autophagy. Oncogenesis. 2019;8(10):59.
Patel NH, Xu J, Saleh T, Wu Y, Lima S, Gewirtz DA. Influence of nonprotective autophagy and the autophagic switch on sensitivity to cisplatin in non-small cell lung cancer cells. Biochem Pharmacol. 1896;2020:113896.
Lin TY, Chan HH, Chen SH, et al. BIRC5/Survivin is a novel ATG12-ATG5 conjugate interactor and an autophagy-induced DNA damage suppressor in human cancer and mouse embryonic fibroblast cells. Autophagy. 2020;16(7):1296–313.
New J, Thomas SM. Autophagy-dependent secretion: mechanism, factors secreted, and disease implications. Autophagy. 2019;15(10):1682–93.
Acknowledgements
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 81472437 and 81672590), National Natural Science Foundation of China Youth Science Foundation (No. 81602438), Doctoral start-up fund of Liaoning Province (Grant No. 201601133) and 345 Talent Project of Shengjing Hospital. The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Jian Gao and Liancheng Zhu contributed equally to this work. Jian Gao and Liancheng Zhu conceived, designed the study, wrote and drafted the manuscript. Jian Gao and Huiyu Zhuang performed the immunohistochemical staining experiment and data analysis. Liancheng Zhu performed the bioinformatics analysis. Bei Lin provided fund support and reviewed the manuscript. All authors read and approved the final version of the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Compliance with Ethics Guidelines
The present study was approved by the Ethical Committee of Shengjing Hospital affiliated to China Medical University (number of approval 2021PS561K).
Disclosures
Jian Gao, Liancheng Zhu, Huiyu Zhuang and Bei Lin all confirm that they have no conflicts of interest to disclose.
Data Availability
The datasets used during the present study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, J., Zhu, L., Zhuang, H. et al. Human Epididymis Protein 4 and Lewis y Enhance Chemotherapeutic Resistance in Epithelial Ovarian Cancer Through the p38 MAPK Pathway. Adv Ther 39, 360–378 (2022). https://doi.org/10.1007/s12325-021-01941-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01941-1