Abstract
Chronic kidney disease (CKD) is a complex disease which affects approximately 13% of the world’s population. Over time, CKD can cause renal dysfunction and progression to end-stage kidney disease and cardiovascular disease. Complications associated with CKD may contribute to the acceleration of disease progression and the risk of cardiovascular-related morbidities. Early CKD is asymptomatic, and symptoms only present at later stages when complications of the disease arise, such as a decline in kidney function and the presence of other comorbidities associated with the disease. In advanced stages of the disease, when kidney function is significantly impaired, patients can only be treated with dialysis or a transplant. With limited treatment options available, an increasing prevalence of both the elderly population and comorbidities associated with the disease, the prevalence of CKD is set to rise. This review discusses the current challenges and the unmet patient need in CKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Chronic kidney disease (CKD) affects a significant proportion of the population and is growing rapidly owing to an increased aging population and prevalence of type 2 diabetes mellitus, obesity, hypertension and cardiovascular disease that contribute towards CKD. |
CKD progression is strongly associated with poor clinical outcomes and has a significant economic burden. |
Despite its high prevalence and the clinical and economic burden of its associated complications, CKD awareness remains profoundly low, in part because CKD is usually silent until its late stages. |
Physician awareness of CKD is critical for the early implementation of evidence-based therapies that can slow progression of renal dysfunction, prevent metabolic complications, and reduce cardiovascular-related outcomes. |
Introduction
Chronic kidney disease (CKD) is a complex and multifaceted disease, causing renal dysfunction and progression to end-stage kidney disease (ESKD) and cardiovascular disease. Complications associated with the disease contribute to the acceleration of CKD progression and risk of cardiovascular-related morbidities.
Despite its high prevalence and the clinical and economic burden of its associated complications, disease awareness remains profoundly low. Worldwide, only 6% of the general population and 10% of the high‐risk population are aware of their CKD statuses [1]. In addition, CKD recognition in primary care settings is also suboptimal, ranging from 6% to 50%, dependent upon primary care specialty, severity of disease, and experience. Awareness of CKD remains low in part because CKD is usually silent until its late stages. However, diagnosis of CKD during the later stages results in fewer opportunities to prevent adverse outcomes. Physician awareness of CKD is critical for the early implementation of evidence-based therapies that can slow progression of renal dysfunction, prevent metabolic complications, and reduce cardiovascular-related outcomes.
Currently CKD is not curable, and management of the disease relies on treatments which prevent CKD progression and cardiovascular disease. Despite available treatments, a residual risk of adverse events and CKD progression remains. This article reviews the challenges associated with CKD and the treatments available for patients, highlighting the unmet need for cardio-renal protection in patients with CKD.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
CKD Prevalence
CKD is a global health problem. A meta-analysis of observational studies estimating CKD prevalence showed that approximately 13.4% of the world’s population has CKD [2]. The majority, 79%, were at late stages of the disease (stage 3–5); however, the actual proportion of people with early CKD (stage 1 or 2) is likely to be much higher since early kidney disease is clinically silent [3].
Prevalence of CKD appears to be growing rapidly both in the UK and in the Western world. Based on the 2012 subnational population projections for England [4], the number of people with CKD stage 3–5 is projected to exceed 4 million by 2036 [5]. This rise in CKD prevalence is due to an increased aging population and prevalence of type 2 diabetes (T2DM), obesity, hypertension and cardiovascular disease that contribute to CKD [6,7,8].
The World Health Organization (WHO) estimated that the annual, global number of deaths caused directly by CKD is 5–10 million [9]. The presence of CKD advances mortality of comorbidities such as cardiovascular diseases, T2DM, hypertension, and infection with human immunodeficiency virus (HIV), malaria and Covid-19, thereby indirectly adding to CKD mortality [9, 10]. A contributing cause of high morbidity and mortality associated with CKD is a lack of awareness of the disease, by both patients and providers [11, 12]. Early stages of CKD are clinically silent and patients have no symptoms. Lack of treatment at this stage allows CKD to progress through to advanced stages of the disease, where patients may present complications and/or cardiovascular-related comorbidities, or ESKD. Raising awareness of CKD is therefore paramount to allow for early intervention and reduce the risk of comorbidities and mortality.
Classification of CKD
In order to better manage CKD and provide better care for patients, the classification of CKD was developed by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative [13] and the international guideline group Kidney Disease Improving Global Outcomes (KDIGO) [14]. CKD stratification is based upon the estimated glomerular filtration rate (eGFR) and albuminuria.
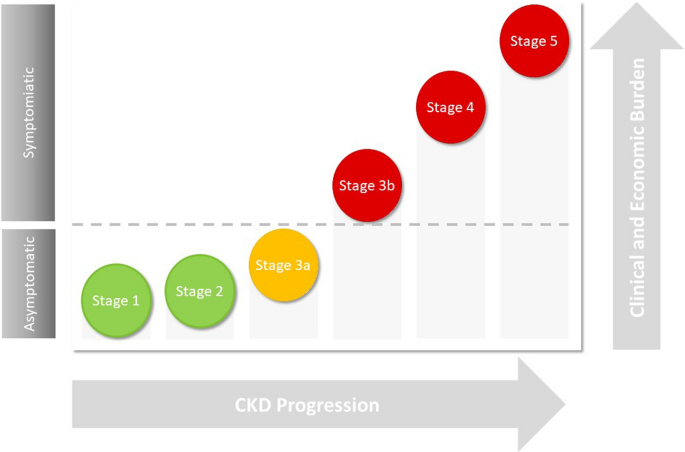
There are six eGFR categories. An eGFR of less than 60 mL/min per 1.73 m2 for more than 3 months is indicative of impaired renal function and the severity of kidney damage increases with decreasing eGFR measurements. Patients with early onset of the disease, stage 1–2, have normal to mild decreased levels of eGFR (60 to ≥ 90 mL/min per 1.73 m2). Patients with stage 3a–3b have mild to moderate decreased levels of eGFR (45–59 mL/min per 1.73 m2, respectively). Severely decreased levels of eGFR, stage 4–5 (15–29 to < 15 mL/min per 1.73 m2, respectively), are indicative of advanced stages of the disease and kidney failure.
Stratification also comprises three categories of albuminuria. Patients with an albumin to creatinine ratio (ACR) of 3 to at most 30 mg/mmol are classified as having microalbuminuria and at moderate risk of adverse outcomes. Those with ACR of greater than 30 mg/mmol are classified as having macroalbuminuria and being severely at risk of developing adverse events [15]. The eGFR and albuminuria categories independently predict adverse outcomes for patients with CKD, and the combination of both increases this risk further [16]. The CKD classification system aids clinicians in carrying out accurate assessments of CKD severity and other complications which helps to inform decisions associated with the management and monitoring of patients [3, 17, 18].
Clinical Burden of CKD
CKD is a complex disease, involving both non-modifiable (e.g. older age, family history and ethnicity) and modifiable risk factors (e.g. T2DM, hypertension and dyslipidaemia) which are responsible for the initiation of early CKD, CKD progression (stage 3–5) and ESKD.
In early stages of CKD (stage 1–2), factors such as hypertension, obesity and T2DM can trigger kidney function impairment. This causes glomerular/interstitial damage and results in impaired glomerular filtration, leading to decreased eGFR and increased albuminuria. At this stage, even though clinical symptoms do not present, the presence of additional risk factors, including hypertension, hyperglycaemia, smoking, obesity, dyslipidaemia and cardiovascular disease, may accelerate CKD progression and result in ESKD.
As the disease progresses, the clinical and economic burden of CKD increases (Fig. 1) as complications such as CKD mineral bone disorder, anaemia, hypertension and hyperkalaemia may occur and advanced stages of CKD, stage 4–5, ensue. Clinical symptoms, such as fatigue, itching of the skin, bone or joint pain, muscle cramps and swollen ankles, feet or hands, are often present at this stage [19]. Further deterioration of kidney function causes tubular and glomerular hypertrophy, sclerosis and fibrosis, leading to a significant reduction in eGFR, extreme albuminuria and kidney failure.
Even though CKD progression may lead to kidney failure and renal death, patients with CKD are more likely to die from cardiovascular-related complications before reaching ESKD [20]. A study using data from a meta-analysis involving 1.4 million individuals found a significant increased risk of cardiovascular-related mortality, even in stage 2 of CKD (eGFR levels < 90 mL/min per 1.73 m2) [16, 21, 22].
As the disease progresses, the risk of cardiovascular disease is markedly increased, such that 50% of patients with late-stage CKD, stage 4–5, have cardiovascular disease. The risk of atrial fibrillation (AF) and acute coronary syndrome (ACS) is doubled in patients with eGFR < 60 mL/min per 1.73 m2. AF is associated with a threefold higher risk of progression to ESKD. The incidence of heart failure (HF) is also threefold greater in patients with eGFR < 60 mL/min per 1.73 m2 compared with > 90 mL/min per 1.73 m2 and HF is associated with CKD progression, hospitalisation and death [23].
The increased risk of cardiovascular disease in patients with CKD is due in part to the traditional risk factors associated with cardiovascular disease such as hypertension, T2DM and dyslipidaemia. For instance, a large observational database linked study (Third National Health and Nutrition Examination Survey (NHANES) III) found a strong association between CKD and T2DM combined and an increased risk of mortality [24]. In this study, the authors observed a 31.1% mortality rate in patients with CKD and diabetes, compared to 11.5% in people with diabetes only. An observational study using both US and UK linked databases showed that the presence of both CKD and T2DM was related to increased risk of major adverse cardiac events (MACE), HF and arrhythmogenic cardiomyopathy (ACM) [25]. This risk was further elevated in older patients with atherosclerotic cardiovascular disease [25]. Similarly, the presence of both CKD and T2DM leads to a significant increased risk of all-cause and cardiovascular-related mortality versus T2DM alone [24].
The direct renal effect on cardiovascular disease is due to generalised inflammatory change, cardiac remodelling, narrowing of the arteries and vascular calcification, both contributing to the acceleration of vascular ageing and atherosclerotic processes, and leading to myocardial infarction, stroke and HF [26].
Together, these studies highlight the strong relationship which exists between CKD progression, number of comorbidities and heightened risk of cardiovascular disease and cardiovascular-related mortality.
Economic Burden of CKD
In addition to the clinical burden, management of CKD also requires significant healthcare resources and utilisation. In 2009–2010, the estimated cost of CKD to the National Health Service (NHS) in England was £1.45 billion [27]. Furthermore, in 2016, US Medicare combined expenditure for CKD and ESKD exceeded $114 billion (£86 billion) [28].
Although estimating the true cost of early CKD is difficult because of the lack of data available for unreported cases, CKD progression is associated with increased healthcare costs [29, 30]. A study by Honeycutt et al. combined laboratory data from NHANES with expenditure data from Medicare and found that costs of CKD management increased with disease progression [29]. Estimated annual medical costs of CKD per person were not significant at stage 1, $1700 at stage 2, $3500 at stage 3 and $12,700 at stage 4.
Healthcare costs associated with early CKD are more likely to be from the sequalae of comorbid disease rather than kidney disease. Hence, patients with CKD stage 1 or 2 are at increased risk of hospitalisation if they also have T2DM (9%), cardiovascular disease (more than twofold), and both cardiovascular disease and T2DM (approximately fourfold) [31].
ESKD accounts for the largest proportion of CKD management costs. In 2009–2010, 50% of the overall CKD cost to NHS (England) was due to renal replacement therapy (RRT), which accounted for 2% of the CKD population [27]. The other 50% included renal primary care costs, such as treatment costs for hypertension and tests, consultation costs, non-renal care attributable to CKD and renal secondary care costs. Approximately £174 million was estimated for the annual cost of myocardial infarctions and strokes associated with CKD [27].
More recently, an economic analysis investigated the burden associated with the management of cardiovascular-related morbidity and mortality in CKD, according to the KDIGO categorisation of both eGFR and albuminuria [15]. Decreased eGFR levels increased both the risk of adverse clinical outcomes and economic costs, and albuminuria elevated this risk significantly. Furthermore, CKD progression correlated with increased CKD management costs and bed days. Stage 5 CKD (versus stage 1 (or without) CKD) per 1000 patient years was associated with £435,000 in additional costs and 1017 bed days.
The significant economic burden associated with CKD progression and ESKD highlights the importance of optimising CKD management and the unmet need for better treatment options in slowing disease progression in this patient population. Thus, early detection and intervention to slow the progression of the disease has the potential to make substantial savings in healthcare costs.
Current CKD Management Strategies
KDIGO and National Institute for Health and Care Excellence (NICE) have produced detailed guidelines for the evaluation and management of CKD [3, 32, 33]. Both recommend implementing strategies for early diagnosis of the disease in order to reduce the risk of cardiovascular disease, attenuate CKD progression and decrease the incidence of ESKD in this patient population. CKD is a complex disease and thus treatment requires a multifaceted approach utilising both non-pharmacological, e.g. diet and exercise regimes and pharmacological interventions such as antihypertensive and antihyperglycemic drugs [34]. There has, however, been no significant breakthrough in this area for over 2 decades.
The effect of lifestyle intervention on reducing disease progression is still unclear, although increased physical activity has been shown to slow the rate of eGFR decline [35] and ESKD progression [36], improve eGFR levels [35] and albuminuria [37], and reduce mortality in patients with CKD [35, 38,39,40]. Similarly, diet regimes such as low-protein diet or Mediterranean diet reduce renal function decline and mortality rate in CKD [41, 42]. Hence, dietary advice is recommended in accordance with CKD severity to control for daily calorie, salt, potassium, phosphate and protein intake [3, 33]. However, patients with consistently elevated serum phosphate levels or metabolic acidosis [low serum bicarbonate levels (< 22 mmol/l)], associated with increased risk of CKD progression and death, may be treated with phosphate binding agents (e.g. aluminium hydroxide and calcium carbonate) or sodium bicarbonate, respectively [3].
To reduce the risk of cardiovascular disease, KDIGO and NICE recommend active lipid management and blood pressure control [33, 43, 44]. In early CKD stages 1 and 2, statins are recommended for all patients over 50 years of age, whilst in stage 3 and advanced stages of the disease, stage 4–5 (eGFR < 60 mL/min per 1.73 m2), a combination of statins and ezetimibe is advised [43].
Management of hypertension includes a target blood pressure of less than 140/90 mmHg for patients with CKD and hypertension and less than 130/80 mmHg for patients with CKD and T2DM, and also in patients with albuminuria [3, 32], alongside blood pressure lowering therapies and renin–angiotensin–aldosterone system (RAAS) blocking agents, such as angiotensin receptor blockers (ARB) or angiotensin-converting enzyme inhibitors (ACEi). As such, RAAS inhibitors (RAASi) are currently recommended to treat patients with diabetes, hypertension and albuminuria in CKD [45]. These RAAS blocking agents confer both renal and cardiovascular protection and are recommended as first-line treatment to treat hypertension in patients with CKD [34, 46].
The clinical benefits of RAASi have been demonstrated in patients with CKD with and without diabetes [47,48,49]. These clinical benefits are in addition to their effects on reducing blood pressure and albuminuria, including a reduction in eGFR decline and a decreased risk of ESKD cardiac-related morbidity and all-cause mortality [47,48,49]. Nevertheless, despite their benefits, RAASi treatment can induce hyperkalaemia, and patients are often advised to reduce RAASi dosage or even discontinue their treatment, which prevents optimum clinical benefits of RAASi therapy being reached. In this instance, combination therapy with potassium binding agents, such as patiromer and sodium zirconium cyclosilicate, may be used alongside RAASi therapy to reduce RAASi-associated hyperkalaemia.
However long-term trials will be required to determine their effect on cardiovascular morbidity and mortality in CKD [50,51,52]. Despite these therapies being the mainstay of CKD management, there is still a residual risk of CKD progression and an unmet need for new treatments.
Novel/Emerging Treatments for CKD Management
Over the last 2 years, novel therapeutic approaches for CKD management have emerged, with particular attention on mineralocorticoid receptor antagonists (MRAs) and sodium–glucose co-transporter 2 (SGLT2) inhibitors. The clinical effectiveness of finerenone, a selective oral, non-steroidal MRA, has recently been demonstrated to lower risks of CKD progression and cardiovascular events in diabetic kidney disease (DKD) [53]. Finerenone is under review for approval by the European Medicines Agency (EMA) and US Food and Drug Administration (FDA).
Of these new and emerging therapies, SGTL2i offers the most clinical benefit with both cardiovascular and renal protective effects, independent of glucose lowering. Clinical trials of SGTL2 in T2DM with and without CKD overall showed a 14–31% reduction in cardiovascular endpoints including hospitalisation for HF and MACE and a 34–37% reduction in hard renal-specific clinical endpoints including a sustained reduction in eGFR, progression of albuminuria and progression to ESKD [54,55,56,57,58]. CREDENCE, was a double-blind, multicentre, randomised trial in diabetic patients with albuminuric CKD (eGFR 30 to < 90 mL/min per 1.73 m2 and ACR ≥ 30 mg/mmol) [57]. In this trial, canagliflozin reduced the relative risk of the composite of ESKD, doubling of serum creatinine and renal-related mortality by 34%, relative risk of ESKD by 34% and risk of cardiovascular-related morbidity, including myocardial infarction and stroke, and mortality.
The SGTL2i dapagliflozin has proven its effectiveness in slowing CKD progression in addition to reducing cardiovascular risk in early stages of CKD. The DECLARE-TIMI58 trial involved 17,160 diabetic patients with established atherosclerotic cardiovascular disease and early-stage CKD (mean eGFR was 85.2 mL/min per 1.73 m2) and were randomised to receive either dapagliflozin or placebo. Following a median follow-up of 4.2 years, there was a significant reduction in renal composite endpoints with dapagliflozin versus placebo, with a 46% reduction in sustained decline of at least 40% eGFR to less than 60 mL/min per 1.73 m2 and a reduction in ESKD (defined as dialysis for at least 90 days, kidney transplantation, or confirmed sustained eGFR < 15 mL/min per 1.73 m2) or renal death.
More recently, these cardiovascular and renal protective effects of SGTLT2i have also been demonstrated in a broad range of patients with more advanced stages of CKD (mean eGFR was 43.1 ± 12.4 mL/min per 1.73 m2) without diabetes [58, 59]. In the DAPA-CKD trial, many patients were without diabetes, including IgA nephropathy, ischemic/hypertension nephropathy and other glomerulonephritis [59]. Patients receiving dapagliflozin had a 39% relative risk reduction in the primary composite outcomes of a sustained decline in eGFR of at least 50%, ESKD and renal- or cardiovascular-related mortality and a 31% relative risk reduction of all-cause mortality compared to placebo [58, 60]. Safety outcomes from clinical trials of dapagliflozin have also shown similar incidences of adverse events in both placebo and dapagliflozin arms [58, 61].
The clinical benefits and safety outcomes from these trials highlight the potential use of SGTL2i in reducing cardiovascular burden and CKD progression in a broad range of CKD aetiologies at early and late stages where there is an unmet need. Currently, SGTL2i class drugs, including canagliflozin, dapagliflozin and empagliflozin, are approved by the US FDA for the treatment of T2DM and, more recently, dapagliflozin and canagliflozin for CKD and DKD respectively [62, 63]. In addition, SGTL2i has been recommended for approval in the European Union (EU) by the Committee for Medicinal Products for Human Use (CHMP) of the EMA, for the treatment of CKD in adults with and without T2D [64]. Hence, there is now a need to raise awareness of the clinical applicability of these drugs in CKD to ensure full utilisation and maximum benefits are met, for both patients and providers.
Conclusion
This narrative review has summarised some of the key challenges associated with CKD. Early stages of the disease are clinically silent which prevents early intervention to slow the progression of the disease and allows progression of CKD and ESKD. At advanced stages of the disease, when clinical symptoms are present, patients with CKD are already at heightened risk of cardiovascular-related morbidity and mortality. Hence, advanced stages of CKD and ESKD are associated with poor outcomes and a significant clinical and economic burden.
At present, there are no treatments to cure CKD; as such, strategies for CKD management have been developed to target the modifiable risk factors in order to reduce cardiovascular disease morbidity in patients with CKD and slow the progression of CKD to ESKD. However, despite available treatment options, residual risk of adverse events and CKD progression remain; hence, an unmet need exists in CKD treatment. SGTL2i have the potential to fill this gap, with recent evidence from clinical trials showing a reduction in cardiovascular and renal adverse endpoints in a broad range of patients with CKD.
References
Ene-Iordache B, Perico N, Bikbov B, et al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health. 2016;4(5):e307–19.
Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease–a systematic review and meta-analysis. PLoS ONE. 2016;11(7):e0158765.
Kidney International Organisation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf. 2012. Accessed May 2021.
Office for National Statistics: Subnational population projections for England: 2012-based. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/bulletins/subnationalpopulationprojectionsforengland/2014-05-29. 2014. Accessed May 2021.
Public Health England: Chronic kidney disease prevalence model. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/612303/ChronickidneydiseaseCKDprevalencemodelbriefing.pdf. 2014. Accessed May 2021.
Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–72.
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305.
Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Primary care Clin Office Pract. 2008;35(2):329–44.
World Health Organization. The global burden of kidney disease and the sustainable development goals. https://www.who.int/bulletin/volumes/96/6/17-206441/en/. 2018. Accessed Apr 2021.
Pecly IMD, Azevedo RB, Muxfeldt ES, et al. COVID-19 and chronic kidney disease: a comprehensive review. J Bras Nefrol. 2021;43(3):383–99.
Tuot DS, Wong KK, Velasquez A, et al. CKD awareness in the general population: performance of CKD-specific questions. Kidney Med. 2019;1(2):43–50.
Plantinga LC, Tuot DS, Powe NR. Awareness of chronic kidney disease among patients and providers. Adv Chronic Kidney Dis. 2010;17(3):225–36.
Levey AS, Coresh J, Bolton K, et al. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266.
Levey AS, Eckardt K-U, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67(6):2089–100.
Darlington O, Dickerson C, Evans M, et al. Costs and healthcare resource use associated with risk of cardiovascular morbidity in patients with chronic kidney Disease: evidence from a systematic literature review. Adv Ther. 2021;38(2):994–1010.
Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–52.
Tonelli M, Muntner P, Lloyd A, et al. Using proteinuria and estimated glomerular filtration rate to classify risk in patients with chronic kidney disease: a cohort study. Ann Intern Med. 2011;154(1):12–21.
Levey AS, Tangri N, Stevens LA. Classification of chronic kidney disease: a step forward. Ann Intern Med. 2011;154(1):65–7.
Abdel-Kader K, Unruh ML, Weisbord SD. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1057–64.
Tuegel C, Bansal N. Heart failure in patients with kidney disease. Heart. 2017;103(23):1848–53.
Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–81.
Van Der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–52.
Kottgen A, Russell SD, Loehr LR, et al. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2007;18(4):1307–15.
Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302–8.
Cherney DZ, Repetto E, Wheeler DC, et al. Impact of cardio-renal-metabolic comorbidities on cardiovascular outcomes and mortality in type 2 diabetes mellitus. Am J Nephrol. 2020;51(1):74–82.
Stevens P, O’Donoghue D, De Lusignan S, Van Vlymen J, Klebe B, Middleton R, et al. Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int. 2007;72(1):92–9.
Kerr M, Bray B, Medcalf J, O’Donoghue DJ, Matthews B. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant. 2012;27(suppl_3):73–80.
Saran R, Robinson B, Abbott KC, et al. US renal data system 2017 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(3):A7.
Honeycutt AA, Segel JE, Zhuo X, Hoerger TJ, Imai K, Williams D. Medical costs of CKD in the Medicare population. J Am Soc Nephrol. 2013;24(9):1478–83.
Smith DH, Gullion CM, Nichols G, Keith DS, Brown JB. Cost of medical care for chronic kidney disease and comorbidity among enrollees in a large HMO population. J Am Soc Nephrol. 2004;15(5):1300–6.
Wang V, Vilme H, Maciejewski ML, Boulware LE. The economic burden of chronic kidney disease and end-stage renal disease. Semin Nephrol. 2016;36:319–30.
National Institute for Health Care Excellence. Chronic kidney disease in adults: assessment and management. https://www.nice.org.uk/guidance/cg182. 2014. Accessed Apr 2021.
National Institute for Health and Care Excellence. Guideline chronic kidney disease. https://www.nice.org.uk/guidance/gid-ng10118/documents/draft-guideline. 2021. Accessed June 2021.
National Institute for Health Care Excellence: Management of chronic kidney disease. https://pathways.nice.org.uk/pathways/chronic-kidney-disease. Accessed Apr 2021.
Chen I-R, Wang S-M, Liang C-C, et al. Association of walking with survival and RRT among patients with CKD stages 3–5. Clin J Am Soc Nephrol. 2014;9(7):1183–9.
Robinson-Cohen C, Littman AJ, Duncan GE, et al. Physical activity and change in estimated GFR among persons with CKD. J Am Soc Nephrol. 2014;25(2):399–406.
Hellberg M, Höglund P, Svensson P, Clyne N. Randomized controlled trial of exercise in CKD—the RENEXC study. Kidney Int Rep. 2019;4(7):963–76.
MacKinnon HJ, Wilkinson TJ, Clarke AL, et al. The association of physical function and physical activity with all-cause mortality and adverse clinical outcomes in nondialysis chronic kidney disease: a systematic review. Therap Adv Chronic Dis. 2018;9(11):209–26.
Beddhu S, Baird BC, Zitterkoph J, Neilson J, Greene T. Physical activity and mortality in chronic kidney disease (NHANES III). Clin J Am Soc Nephrol. 2009;4(12):1901–6.
Clarke AL, Zaccardi F, Gould DW, et al. Association of self-reported physical function with survival in patients with chronic kidney disease. Clin Kidney J. 2019;12(1):122–8.
Chauveau P, Aparicio M, Bellizzi V, et al. Mediterranean diet as the diet of choice for patients with chronic kidney disease. Nephrol Dial Transplant. 2018;33(5):725–35.
Rhee CM, Ahmadi SF, Kovesdy CP, Kalantar-Zadeh K. Low-protein diet for conservative management of chronic kidney disease: a systematic review and meta-analysis of controlled trials. J Cachexia Sarcopenia Muscle. 2018;9(2):235–45.
Wanner C, Tonelli M. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85(6):1303–9.
National Institute for Health and Care Excellence. Cardiovasvcular disease: risk assessment and reduction, including lipid modification. https://www.nice.org.uk/guidance/cg181/resources/cardiovascular-disease-risk-assessment-and-reduction-including-lipid-modification-pdf-35109807660997. 2014. Accessed Apr 2021.
de Boer IH, Caramori ML, Chan JC, et al. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98(4):S1–115.
National Collaborating Centre for Chronic Conditions. Chronic kidney disease: national clinical guideline for early identification and management in adults in primary and secondary care. London: Royal College of Physicians; 2008.
Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease: a meta-analysis of patient-level data. Ann Intern Med. 2001;135(2):73–87.
Evans M, Bain SC, Hogan S, Bilous RW. Irbesartan delays progression of nephropathy as measured by estimated glomerular filtration rate: post hoc analysis of the Irbesartan Diabetic Nephropathy Trial. Nephrol Dial Transplant. 2012;27(6):2255–63.
Xie X, Liu Y, Perkovic V, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67(5):728–41.
Wai H-T, Meah N, Katira R. Novel potassium binders: a clinical update. https://bjcardio.co.uk/2021/04/novel-potassium-binders-a-clinical-update-3/. Accessed June 2021.
National Institute for Health and Care Excellence. Sodium zirconium cyclosilicate for treating hyperkalaemia. Technology appraisal guidance TA599. https://www.nice.org.uk/guidance/TA599 (2019). Accessed April 2021.
National Institute for Health and Care Excellence. Patiromer for treating hyperkalaemia. https://www.nice.org.uk/guidance/ta623/chapter/1-Recommendations. 2020. Accessed May 2021.
Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–29.
Zinman B, Wanner C, Lachin J. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57.
Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306.
Heerspink HJ, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46.
Wheeler DC, Stefansson BV, Batiushin M, et al. The dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial: baseline characteristics. Nephrol Dial Transplant. 2020;35(10):1700–11.
Heerspink HJ, Sjöström CD, Jongs N, et al. Effects of dapagliflozin on mortality in patients with chronic kidney disease: a pre-specified analysis from the DAPA-CKD randomized controlled trial. Eur Heart J. 2021;42(13):1216–27.
McMurray JJ, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008.
U.S. Food & Drug Administration (FDA). FDA approves treatment for chronic kidney disease. https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-chronic-kidney-disease. 2021. Accessed May 2021.
Janssen Pharmaceutical of Johnson & Johnson. U.S. FDA approves INVOKANA® (canagliflozin) to treat diabetic kidney disease (DKD) and reduce the risk of hospitalization for heart failure in patients with type 2 diabetes (T2D) and DKD. https://www.prnewswire.com/news-releases/us-fda-approves-invokana-canagliflozin-to-treat-diabetic-kidney-disease-dkd-and-reduce-the-risk-of-hospitalization-for-heart-failure-in-patients-with-type-2-diabetes-t2d-and-dkd-300927348.html. 2019. Accessed May 2021.
European Medicines Agency. Forxiga: Summary of opinion (post authorisation). https://www.ema.europa.eu/en/medicines/human/EPAR/forxiga. 2021. Accessed May 2021.
Acknowledgements
Funding
This manuscript was supported by a grant from AstraZeneca UK Ltd. in respect of medical writing and publication costs (the journal’s Rapid Service and Open Access Fees). AstraZeneca has not influenced the content of the publication, and has reviewed this document for factual accuracy only.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
All authors have been involved in design of this review. Marc Evans, Ruth D. Lewis, and Angharad R. Morgan produced the primary manuscript. All authors have contributed to the drafting and revision of the manuscript and have approved the final version for publication. Marc Evans is responsible for the integrity of the work as a whole.
Disclosures
Marc Evans reports honoraria from AstraZeneca, Novo Nordisk, Takeda and NAPP, and research support from Novo Nordisk outside the submitted work. Ruth D. Lewis and Angharad R Morgan are employees of Health Economics and Outcomes Research Ltd., Cardiff, UK who received fees from AstraZeneca in relation to this study. Martin B. Whyte reports investigator-led research grants from Sanofi, Eli Lilly and AstraZeneca and personal fees from AstraZeneca, Boehringer Ingelheim and MSD outside the submitted work. Wasim Hanif reports grants and personal fees from AstraZeneca, grants and personal fees from Boerhinger Inglhiem, grants and personal fees from NAPP, from MSD, outside the submitted work. Stephen C. Bain reports personal fees and other from Abbott, personal fees and other from AstraZeneca, personal fees and other from Boehringer Ingelheim, personal fees and other from Eli Lilly, personal fees and other from Merck Sharp & Dohme, personal fees and other from Novo Nordisk, personal fees and other from Sanofi-aventis, other from Cardiff University, other from Doctors.net, other from Elsevier, other from Onmedica, other from Omnia-Med, other from Medscape, other from All-Wales Medicines Strategy Group, other from National Institute for Health and Care Excellence (NICE) UK, and other from Glycosmedia, outside the submitted work. PAK reports personal fees for lecturing from AstraZeneca, Boehringer Inglhiem, NAPP, MundiPharma and Novo Nordisk outside the submitted work. Sarah Davies has received honorarium from AstraZeneca, Boehringer Ingelheim, Lilly, Novo Nordisk, Takeda, MSD, NAPP, Bayer and Roche for attending and participating in educational events and advisory boards, outside the submitted work. Umesh Dashora reports personal fees from AstraZeneca, NAPP, Sanofi, Boehringer Inglhiem, Lilly and Novo Nordisk, outside the submitted work. Zaheer Yousef reports personal fees from AstraZeneca, personal fees from Lilly, personal fees from Boehringer Ingelheim and personal fees from Novartis outside the submitted work. Dipesh C. Patel reports personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Eli Lilly, non-financial support from NAPP, personal fees from Novo Nordisk, personal fees from MSD, personal fees and non-financial support from Sanofi outside the submitted work. In addition, DCP is an executive committee member of the Association of British Clinical Diabetologists and member of the CaReMe UK group. W. David Strain holds research grants from Bayer, Novo Nordisk and Novartis and has received speaker honoraria from AstraZeneca, Bayer, Bristol-Myers Squibb, Merck, NAPP, Novartis, Novo Nordisk and Takeda. WDS is supported by the NIHR Exeter Clinical Research Facility and the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) for the South West Peninsula.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Evans, M., Lewis, R.D., Morgan, A.R. et al. A Narrative Review of Chronic Kidney Disease in Clinical Practice: Current Challenges and Future Perspectives. Adv Ther 39, 33–43 (2022). https://doi.org/10.1007/s12325-021-01927-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01927-z