Abstract
Introduction
Both radiofrequency (RF) and cryoballoon (CB) ablation are treatment options for persistent atrial fibrillation (PsAF). An important recent innovation in RF ablation is Ablation Index (AI), known also as the VISITAG SURPOINT™ Module, a composite lesion quality marker whose use has been shown to significantly reduce the incidence of acute and late pulmonary vein (PV) reconnection and the recurrence of atrial arrhythmias in PsAF. Due to a lack of direct comparative evidence between the latest generations of technologies, there is uncertainty regarding the best treatment option in PsAF. The objective of the present study was to conduct a matching-adjusted indirect treatment comparison (MAIC) using individual patient-level data (IPD) to assess the comparative effectiveness of the THERMOCOOL SMARTTOUCH™ Catheter or the THERMOCOOL SMARTTOUCH™ SF Catheter with AI/VISITAG SURPOINT™ Module (STAI) versus the second-generation CB catheter (Arctic Front Advance™; herein referred to as CB) with respect to 12-month atrial arrhythmia recurrence, fluoroscopy time, and procedural efficiency.
Methods
IPD for STAI were obtained from four investigator-initiated studies and were pooled. Comparable CB studies identified from a systematic literature review were also pooled. In the absence of a common treatment arm between STAI and CB studies, an unanchored MAIC was conducted. The primary analysis compared the pooled STAI IPD to the pooled CB cohort, with corrections for differences across trials, including eligibility criteria and patient baseline characteristics. Scenario and sensitivity analyses were conducted to assess the robustness of the primary analysis.
Results
In the primary analysis, which was adjusted for left atrial diameter (LAD), age, diabetes, and sex, STAI was associated with a statistically significant 65% relative reduction in the rate of arrhythmia recurrence compared to CB at 12-month follow-up (HR 0.35; 95% CI 0.23, 0.52). STAI was associated with shorter total fluoroscopy time than CB but longer procedure time. Results were consistent across scenario and sensitivity analyses.
Conclusion
Radiofrequency ablation with AI significantly reduced atrial arrhythmia recurrence at 12-month follow-up and fluoroscopy time compared to CB, with longer procedure times.
Similar content being viewed by others
Catheter ablation is an effective rhythm control strategy that is recommended by guidelines for the management of persistent atrial fibrillation (PsAF). |
Catheters for radiofrequency (RF) and cryoballoon (CB) ablation are approved for use in PsAF; however, due to a lack of direct comparative evidence between the latest generations of technologies, there is uncertainty regarding the best treatment option in PsAF. |
We conducted an unanchored, matching-adjusted indirect treatment comparison using individual patient-level data to assess the comparative effectiveness of Ablation Index-guided RF ablation and cryoablation using the second-generation CB in terms of 12-month atrial arrhythmia recurrence, fluoroscopy time, and procedural efficiency. |
Results showed that Ablation Index-guided RF ablation was associated with a statistically significant 65% reduction in the rate of arrhythmia recurrence compared to CB at 12-month follow-up, as well as shorter fluoroscopy time, and longer procedure time. |
To the best of our knowledge, this study is the first to provide robust, pooled, comparative evidence for the latest generations of catheter ablation devices used in the treatment of PsAF. |
Introduction
Catheter ablation is an effective, guideline-recommended rhythm control strategy for the management of persistent atrial fibrillation (PsAF) [1,2,3]. The primary strategy for ablation is pulmonary vein isolation (PVI); however, more extensive ablation may be required in PsAF, including ablation of linear lesions in the atria, isolation of the left atrial appendage or the superior vena cava, and ablation of complex fractionated electrograms, rotors, nonpulmonary foci, or ganglionated plexi [2]. Both radiofrequency (RF) and cryoballoon (CB) ablation may be used in PsAF; however, uncertainty remains regarding their comparative effectiveness.
Two large, prospective, multicenter, Food and Drug Administration (FDA)-regulated trials have recently demonstrated the safety and effectiveness of CB and RF ablation in PsAF [4, 5]. The STOP Persistent Atrial Fibrillation (STOP-AF) trial evaluated the efficacy and safety of PVI-only cryoablation in PsAF using the Arctic Front Advance™ (Medtronic, Minneapolis, MN, USA) CB. Freedom from atrial arrhythmia was reported to be 54.8% [95% confidence interval (CI) 46.7–62.1] at 12 months [4]. The PRECEPT trial evaluated the safety and efficacy of RF ablation in PsAF using the contact-force sensing THERMOCOOL SMARTTOUCH™ SF Catheter (Biosense Webster, Irvine, CA, USA) guided by the CARTO VISITAG™ Module (Biosense Webster) using an individualized treatment approach including PVI with ablation of additional targets permitted at the investigator’s discretion. Overall freedom from documented symptomatic atrial arrhythmia was reported to be 80.4% at 15 months after ablation [5]. Naïve comparison of results from these studies suggest that ablation with point-by-point RF catheters may provide improved efficacy over cryoablation in PsAF patients. The latest innovation in RF ablation, the CARTO VISITAG™ Module with Ablation Index (AI) (also known as the VISITAG SURPOINT™ Module; Biosense Webster), is a lesion-quality marker that has been shown to significantly reduce the incidence of acute and late pulmonary vein (PV) reconnection and to reduce the recurrence of atrial arrhythmias in PsAF patients when used in combination with the THERMOCOOL SMARTTOUCH™ Catheter or THERMOCOOL SMARTTOUCH™ SF Catheter [6,7,8,9].
Currently, there are no randomized head-to-head trials that have directly compared the effectiveness of CB to RF ablation with AI guidance in PsAF. Thus, indirect treatment comparison methods, such as matching-adjusted indirect comparisons (MAICs), are required to balance trial populations for fair comparison [10, 11]. MAICs use individual patient-level data (IPD) to match the patient cohort in one study to the eligibility criteria of another, and then to adjust for between-study differences in patient populations to reduce differences in prognostic factors and treatment-effect modifiers between the two groups.
The primary objective of the present study was to assess the comparative effectiveness of the THERMOCOOL SMARTTOUCH™ Catheter or THERMOCOOL SMARTTOUCH™ SF Catheter with AI (STAI) versus the second-generation CB catheter (i.e., Arctic Front Advance™) in terms of 12-month atrial arrhythmia recurrence. The secondary objective was to compare fluoroscopy time and procedural efficiency between technologies.
Methods
Identification of Studies for MAIC Analyses
Individual patient-level data from four investigator-initiated studies on STAI that included patients with PsAF were available for analysis [6,7,8, 12]. The first study by Hussein et al. [6] was a prospective, single-arm registry that included 40 PsAF patients. A second study by Hussein et al. [7] was a prospectively collected, propensity score-matched analysis comparing STAI to a historical control group and included 31 PsAF patients treated with STAI. The study by Solimene et al. [8] was a prospective, single-arm registry that included 32 PsAF patients treated with STAI. Finally, the study by Stabile et al. [12] was also a prospective registry, and included 96 PsAF patients treated with STAI. Across all four studies, a total of 191 PsAF patients treated with STAI were available for analysis.
A systematic literature review was conducted to identify CB studies in patients with PsAF published between January 2010 and May 2019 (Supplementary Material, Table S1). An updated literature search based on the search strategy and methodology from the systematic literature review was conducted on January 19, 2021 to identify CB studies in patients with PsAF published after May 2019. To ensure a sufficient sample size for robust matching and adjustment of STAI IPD to the CB cohort, prospective studies were eligible for inclusion if they enrolled a minimum of 50 patients with drug-refractory, symptomatic PsAF who received first-time ablation and used the second-generation Arctic Front Advance™ cryoablation catheter (Supplementary Material, Table S1). The Arctic Front Advance™ ablation catheter was selected as the most relevant comparator because it represented the standard of care for cryoablation catheters at the time of the analysis. Outcomes of interest included time-to-event data (i.e., Kaplan–Meier curves) on freedom from atrial arrhythmias or recurrence of atrial arrhythmias with a minimum follow-up period of 12 months (excluding events that occurred during a 3-month blanking period), total fluoroscopy time, and overall procedural time. Non-English-language articles were excluded.
Data Extraction
Data extracted (where available) included study design, sample size, atrial fibrillation (AF) type (i.e., paroxysmal AF, PsAF, or long-standing PsAF), and patient baseline characteristics [e.g., age, sex, diabetes, left atrial diameter (LAD), left ventricular ejection fraction (LVEF), body mass index, CHADS score, heart disease, heart failure, hypertension, and stroke or transient ischemic attack]. The primary outcome of interest was freedom from atrial arrhythmias or recurrence of atrial arrhythmias at least 12 months after AF catheter ablation, as defined by the study authors (Supplementary Material, Table S2). Atrial arrhythmias were defined as AF, atrial flutter, or atrial tachycardia, as defined by the study authors. Secondary endpoints were total fluoroscopy time and overall procedure time, as defined by the study authors.
Methods for MAIC Analyses
The primary analysis aimed to compare the pooled STAI IPD from all four STAI trials to the pooled CB cohort. Pooling the STAI IPD from all four STAI trials increased the sample size available for adjusting to the pooled CB cohort to reduce residual imbalances between treatments. Three electrophysiologists (G.S., A.H., D.G.) provided a priori and independent rankings of potential prognostic factors and treatment-effect modifiers that were reported in the STAI and CB trials, and were considered relevant with regard to catheter ablation of PsAF. Average rankings were calculated and used to order potential factors for adjustment in scenario analyses (Supplementary Material, Table S3).
An unanchored MAIC was conducted between the pooled STAI IPD and the pooled CB cohorts because of a lack of a common comparator between studies. MAIC analyses were conducted using methods outlined by Phillippo et al. [11], and detailed methods have been previously described [13]. Briefly, IPD from the STAI studies were matched to the CB studies by removing STAI patients who would have not met the eligibility criteria of the CB studies. The resulting set of matched patients from the STAI IPD were then reweighted on the basis of a propensity score method-of-moments estimation. Adjustment of the primary analysis was limited to the set of factors that were reported in all studies. The primary analysis for each comparison of datasets was the analysis that adjusted for the most factors.
To estimate absolute events associated with cryoablation, reconstructed IPD representing time-to-event outcomes from the CB studies were derived from published Kaplan–Meier curves using the Guyot algorithm [14]. After matching and reweighting of STAI IPD, the reconstructed CB IPD and STAI IPD were combined to facilitate estimating the comparative effectiveness of STAI versus CB for recurrence of atrial arrhythmias, and to assess differences in fluoroscopy time and procedural efficiency between treatments. Comparative effectiveness estimates were obtained by fitting a mixed-effects Cox proportional hazards regression model with random study-level effects using the reweighted IPD set [15]. Estimates of absolute effects were reported separately for STAI and CB as the cumulative proportion of patients who had an arrhythmia recurrence at 12-month follow-up. Estimates of relative effects were reported as a hazard ratio (HR) and 95% CIs. CIs that do not cross unity (i.e., 1) were considered statistically significant. Pooled means and standard deviations (SDs) for CB procedure time and fluoroscopy time were calculated using a fixed effect inverse-variance weighted meta-analysis. For STAI, matched and adjusted estimates of the mean (SD) of procedure time were derived from IPD. Estimates of relative effects were reported as mean differences (MD) and 95% CIs; CIs that do not cross 0 were considered statistically significant. Statistical analyses were conducted using the R Statistical Software [16].
Scenario and Sensitivity Analyses
Scenario and sensitivity analyses were conducted to assess the robustness of the primary analyses and to explore the impact of adjusting for additional factors. Scenario analyses were performed wherein the MAICs were conducted iteratively, removing one factor at a time in order of least importance. Sensitivity analyses included one-at-a-time factor adjustments, where a single factor was adjusted in MAIC, and the exclusion of one study [4] from the pooled CB cohort to assess the potential impact of between-study variability with respect to participant baseline disease status and outcome definition. Additionally, to permit adjustment of LVEF, MAIC estimates from a series of MAICs, each comparing one STAI study to the pooled CB studies, were synthesized into a single comparative efficacy estimate. This permitted adjustment for LVEF in the comparison of the Solimene et al. STAI study and the pooled CB studies and, therefore, partial adjustment of LVEF in the pooled analysis. The single comparative efficacy estimate was synthesized using a fixed effect inverse-variance weighted meta-analysis [17].
Compliance with Ethical Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
Literature Review Results
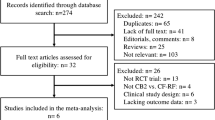
A total of 2813 citations were identified (Fig. 1). Of a total of 325 citations identified for full-text review, 4 were eligible for inclusion in the present analysis [4, 18,19,20]. Reasons for exclusion included study design (e.g., retrospective studies), population (e.g., paroxysmal AF, long-standing PsAF), irrelevant intervention or comparator, or if the study did not report the outcomes of interest.
CB SLR PRISMA flow diagram (an SLR was conducted to identify CB studies in patients with PsAF published between January 2010 to May 2019. An updated literature search based on the original search strategy was conducted on January 19, 2021 to identify relevant studies published after May 2019). CB second-generation cryoballoon, n sample size, PsAF persistent atrial fibrillation, SLR systematic literature review
Study Characteristics and Prognostic Factors and Treatment-Effect Modifiers
A total of 389 patients with PsAF were pooled from four CB trials [4, 18,19,20]. The mean (SD) age of the pooled CB cohort was 63.1 (10.2) years. The proportion of patients who were male (68.3–74.3%), the prevalence of diabetes (5–12.1%), left atrial diameter (LAD) (42–47.1 mm), and LVEF (53.8–57.4%) varied across individual CB study populations (Table 1). After matching on AF type, a total of 191 patients with PsAF were pooled from four STAI studies for subsequent analysis. Three patients from this cohort were missing data on fluoroscopy time and were excluded from the fluoroscopy time analysis. The mean age of the pooled STAI cohort was 61.3 (SD 9.4) years and trials differed with regard to LAD (43.0–46.5 mm), the proportion of patients who were male (54.2–80.6%), and the prevalence of diabetes (4.2–22.6%) (Table 1). Mean LVEF was reported in two of the four STAI trials and was 56%. The proportion of patients who were receiving amiodarone prior to the ablation procedure was reported in one study (Table 1).
Before adjusting, several differences in prognostic factors and treatment-effect modifiers were observed among patients in the pooled CB cohort compared to those in the pooled STAI cohort. After adjusting, the means and proportions of commonly reported prognostic factors and treatment-effect modifiers from the STAI IPD were balanced with those in the pooled CB cohort (Table 2).
MAIC Results
Rate of Atrial Arrhythmia Recurrence
Overall, estimates of comparative effectiveness between the pooled STAI cohort and the pooled CB cohort showed that STAI was associated with a significantly lower rate of AF recurrence than CB. Naïve comparison of the pooled STAI cohort and the pooled CB cohort showed lower cumulative probabilities of arrhythmia recurrence for STAI (18%) than CB (41%) at 12-month follow-up, which corresponded to a statistically significant 61% reduction in the rate of atrial arrhythmia recurrence with STAI compared to CB (HR 0.39; 95% CI 0.27, 0.56). In the primary analysis that adjusted for the common prognostic factors of LAD, age, diabetes, and sex, STAI was associated with a statistically significant 65% reduction in the rate of atrial arrhythmia recurrence compared to CB (HR 0.35; 95% CI 0.23, 0.52) (Figs. 2, 3). STAI continued to be associated with a statistically significant reduction in the rate of atrial arrhythmia recurrence compared to CB in scenario analyses that adjusted for LAD, age, and sex (65% reduction; HR 0.35; 95% CI 0.23, 0.52) and LAD and age (66% reduction; HR 0.34; 95% CI 0.23, 0.51).
Hazard ratio for arrhythmia recurrence (pooled STAI IPD versus pooled CB cohorts). Forest plot for the pooled STAI cohort versus the pooled CB cohort for arrhythmia recurrence with STAI compared to CB at 12-month follow-up. HR < 1 represents lower recurrence with STAI than CB, whereas HR > 1 represents greater recurrence with STAI than CB. AF atrial fibrillation, CB second-generation cryoballoon, CI confidence interval, ESS effective sample size, HR hazard ratio, IPD individual patient-level data, N sample size, SD standard deviation, STAI THERMOCOOL SMARTTOUCH™/THERMOCOOL SMARTTOUCH™ SF Catheter with AI
Furthermore, STAI was associated with statistically significant reductions in the rate of atrial arrhythmia recurrence compared to CB across all univariate MAICs (i.e., adjusted one factor at a time) (Supplementary Material, Figure S2).
Fluoroscopy Time and Procedure Time
In all analyses, total fluoroscopy time was found to be significantly shorter with STAI than with CB. Naïve comparison of the pooled STAI IPD cohort and the pooled CB cohort showed that mean fluoroscopy time was significantly shorter with STAI than with CB (MD − 7.2; 95% CI − 10.6 to − 3.7). In the primary analysis that adjusted for LAD, age, sex, and diabetes, mean fluoroscopy time was 7.0 min shorter with STAI than with CB (MD − 7.0 min; 95% CI − 10.5 to − 3.5) (Fig. 4). Furthermore, fluoroscopy time was significantly shorter with STAI than with CB across all univariate MAICs (Supplementary Material, Figure S3).
Mean differences in fluoroscopy time and procedure time (pooled STAI IPD vs. pooled CB cohorts). Mean difference between the pooled STAI IPD cohort versus the pooled CB cohort for fluoroscopy time (A) and procedure time (B). Mean difference < 0 favors STAI, whereas MD > 0 favors CB. CB second-generation cryoballoon, ESS effective sample size, IPD individual patient-level data, MD mean difference, N sample size, SD standard deviation, STAI THERMOCOOL SMARTTOUCH™/THERMOCOOL SMARTTOUCH™ SF Catheter with AI
Overall procedure time was found to be significantly longer with STAI than with CB. Naïve comparison of the pooled STAI IPD cohort and the pooled CB cohort showed that procedure time was approximately 52 min longer with STAI than with CB (MD 52.2 min; 95% CI 16.0, 88.3). After adjustment for the common prognostic factors of LAD, age, sex, and diabetes procedure time continued to be significantly longer with STAI than with CB (MD 55.3 min; 95% CI 20.5, 90.1) (Fig. 4). Results were similar across univariate MAICs (Supplementary Material, Figure S3).
Sensitivity Analyses
A sensitivity analysis that excluded one study [4] from the pooled CB cohort, and adjusted for the common prognostic factors of LAD, age, diabetes, and sex, showed that STAI continued to be associated with a statistically significant reduction in the rate of atrial arrhythmia recurrence compared to CB at 12-month follow-up (HR 0.38; 95% CI 0.25, 0.59) (Supplementary Material, Table S4). Another sensitivity analysis that adjusted for the common prognostic factors, as well as partial adjustment for LVEF (via a meta-analysis of pairwise MAICs approach) also showed that STAI was associated with a statistically significant reduction in the rate of atrial arrhythmia recurrence compared to CB at 12-month follow-up (HR 0.43; 95% CI 0.27, 0.69) (Supplementary Material, Table S4). In both sensitivity analyses, mean differences in total fluoroscopy time and overall procedure time between the STAI IPD cohort and the CB cohort were similar to those in the primary analysis (Supplementary Material, Table S4).
Discussion
Both CB and STAI are successful in the ablation of PsAF, with 12-month recurrence rates reported in the studies included in this analysis of 39–45% for cryoablation and 17–20% for STAI [4, 6,7,8, 12, 18,19,20]. However, their comparative efficacy in this AF subtype is unknown. We conducted an MAIC to compare the effectiveness of STAI to that of CB for recurrence of atrial arrhythmias 12 months after catheter ablation in patients with PsAF, and found that ablation using STAI was associated with significant reductions in recurrence of atrial arrhythmias compared to CB before and after matching and adjusting for differences between studies and patient characteristics. STAI was also associated with shorter fluoroscopy times than CB and longer procedure times. These results were robust across multiple scenario and sensitivity analyses.
Contemporary meta-analytic studies have sought to assess the comparative efficacy of RF and CB ablation for AF [21,22,23]; however, these analyses included mixed AF types and/or generations of ablation technologies, precluding comparison of the latest generations of RF and CB technologies available for the treatment of PsAF. A meta-analysis by Liu and colleagues [23] is the first to compare the efficacy and safety of cryoablation and RF ablation in PsAF ablation. The analysis found that there was no difference in terms of freedom from atrial arrhythmia between cryoablation and RF ablation (RR 1.04; 95% CI 0.93–1.15; P = 0.52) [23]. Importantly, studies included in the analysis differed with respect to study design, intervention (i.e., mixed generation and/or type of catheter), and ablation strategy (i.e., PVI alone, PVI plus additional ablations). Further to the mixed use of first- and second-generation CB catheters and/or contact force-sensing and standard open irrigated catheters, none of the included studies used ablation index-guided RF ablation.
In the absence of comparative evidence for STAI and CB, the present analysis evaluated the recurrence of atrial arrhythmias following STAI and CB 12 months after catheter ablation in patients with PsAF, with correction for differences in AF type and patient baseline characteristics. To the best of our knowledge, the present analysis is the first to provide robust, pooled, comparative evidence for the latest generations of catheter ablation devices used in the treatment in PsAF, namely STAI and CB.
In a systematic review of AF ablation technology, the use of AI with point-by-point RF ablation was associated with higher acute success rates and lower acute reconnection rates than contact force-guided RF ablation, which translated into less recurrence of atrial arrhythmias at 12 months [24]. Non-PV triggers are recognized as being important in non-paroxysmal AF, and there is often a need for additional lines of ablation and/or lesion sets beyond PVI alone [2, 25,26,27,28]; however, the evidence for this is not well established, and uncertainty regarding the optimal ablative approach in this population remains [29]. In the present analysis, ablation beyond PVI was permitted at the operator’s discretion in all but one of the STAI studies, which may have contributed to a more robust ablation strategy that lowered the rate of recurrence observed with STAI compared to CB. One of the STAI studies reported 67% (n = 21) of patients underwent cavotricuspid isthmus ablation or left atrial posterior wall ablation with a box lesion set [6]. One study reported 8% (n = 3) of patients received cavotricuspid isthmus ablation [7]; in the other study, one patient with PsAF received a posterior wall box lesion [12]. In contrast, three of the CB studies reported using a PVI-only approach [4, 19, 20], and one reported 7.9% (n = 8) of patients underwent right atrial flutter ablations using RF energy [18]. Emerging evidence suggests that posterior wall isolation in conjunction with PVI using CB may be superior to PVI alone in patients with PsAF [30,31,32]. However, non-PV ablation with the CB has been poorly validated, and the device is not currently indicated for posterior wall ablation. Additionally, at the time of this analysis, a standardized endpoint for successful electrical isolation of the posterior wall using this approach was unavailable. Therefore, to focus the comparison of technologies on how they are currently used in clinical practice, this approach was not considered in the present analysis.
In line with recent guidelines, PsAF was defined in most studies as sustained episodes of AF lasting > 7 days and < 12 months. However, in the STOP PsAF trial, PsAF was defined as continuous PsAF episodes lasting > 7 days but < 6 months [4], indicating that patients may have differed from those enrolled in other studies with respect to baseline disease status. Furthermore, as is typical for an FDA-regulated trial, the primary efficacy endpoint at 12 months used in the STOP PsAF trial was clearly defined by the following four criteria: acute procedural success (defined as PVI achieved by cryoablation), freedom from atrial arrhythmia recurrence after a 90-day blanking period, freedom from repeat ablation after the blanking period, and no class I or III antiarrhythmic drug (AAD) initiation or dose increase after the blanking period [4]. As such, the efficacy outcome may have differed from those used in the other included studies. For example, although some studies permitted the continued use of AAD therapy at the clinicians’ discretion, details regarding change and/or increase in AAD dosage after the blanking period were not clearly reported. Additionally, a focal 8-mm tip cryoablation catheter was permitted as an adjunctive tool to complete PVI in the STOP PsAF trial, whereas the other CB studies did not report focal touch-up with either a cryoablation or RF catheter. Despite these differences, a sensitivity analysis that was conducted to assess the impact of removal of this study found that results were consistent with the primary analysis.
Another key difference across trials is that all patients in the PRAISE study underwent a protocol-mandated repeat procedure to assess for late reconnection and to re-isolate PVs, as needed [7]. Late PV reconnection was identified in 7% (n = 11) of PVs, and in 22% (n = 8) of patients; all reconnected PVs were successfully re-isolated. Freedom from AF recurrence was reported to be lower among patients who required redo ablation for PV reconnection than among those who had no reconnection; however, the difference was not statistically significant. Nonetheless, this protocol-mandated repeat procedure may have contributed to a lower rate of AF recurrence in the trial, which may have impacted the overall effectiveness of the pooled STAI dataset.
Consistent with the ablation strategy of PVI plus additional non-PV targets in RF ablation compared to PVI-only cryoablation, procedure time was found to be longer with STAI than CB. Notably, in all STAI studies, resumption of left atria to PV conduction was evaluated for at least 20 min after ablation, and two studies reported administering an adenosine challenge to unmask dormant PV reconnection if no spontaneous reconnection was seen. This additional time during the procedure was a major contributor to the overall difference in procedure time. None of the CB studies reported observing a waiting period to observe PV reconnection, and only one of the CB studies reported that investigators were permitted to test acute PVI with isoproterenol and/or adenosine [4]. Additionally, STAI was associated with approximately half the fluoroscopy time than that of CB. This is an important finding, given that reductions in fluoroscopy time and/or radiation dose are important concerns, as fluoroscopy exposure during catheter ablation is a known health hazard to both patients and operators [33].
Unanchored MAICs are subject to a number of inherent limitations as they are based on several methodological assumptions. For the present analysis, an unanchored MAIC approach was taken as the best possible approach due to lack of a common comparator between STAI and CB studies. Conduct of an unanchored MAIC assumes that all relevant prognostic factors and treatment effect modifiers have been adequately adjusted for. However, since it is rarely possible to conduct a comparison that accounts for all potential differences in prognostic factors or treatment effect modifiers, this assumption is considered very difficult to meet. Additionally, results may have been impacted by the inability to correct for differences in study eligibility criteria, either because of a lack of reporting of such criteria or because the data collected lacked sufficient detail to do so. As such, an important limitation of the present analysis was the inability to adjust for differences that were not explained by reported eligibility criteria and/or patient characteristics across trials. For example, the results may have been impacted by the inability to adjust for variability in the method of monitoring of atrial arrhythmias across studies (Supplementary Material, Table S5). This may affect the robustness or generalizability of the analysis results. Studies of STAI and CB were pooled to increase the sample size and to provide a larger evidence base within the MAIC. As a result, the number of factors available for adjustment were limited to those reported across all trials. The primary analysis had sufficient sample size to adjust for four prognostic factors (i.e., LAD, age, sex, and diabetes). Although AF duration was identified as a relevant prognostic factor in PsAF, it was unavailable for adjustment due to substantial variation and/or lack of reporting of this factor across studies (Supplementary Material, Table S6). Despite this limitation, a range of scenario analyses using different sets of factors resulted in similar conclusions regarding the comparative efficacy of STAI compared with CB in PsAF. A sensitivity analysis that adjusted for an additional prognostic factor (i.e., LVEF) was consistent with the primary analyses, albeit resulted in smaller effect estimates and lower effective sample sizes.
Conclusions
Using an unanchored MAIC approach, catheter ablation of PsAF using the THERMOCOOL SMARTTOUCH™ Catheter or THERMOCOOL SMARTTOUCH™ SF Catheter guided by the CARTO VISITAG™ Module with the AI/ VISITAG SURPOINT™ Module was associated with a significant reduction of 12-month atrial arrhythmia recurrence and fluoroscopy time compared to second generation CB, but with longer procedure times. Despite limitations inherent to unanchored MAIC, results were consistent across multiple scenario and sensitivity analyses, providing further evidence to inform decision makers on appropriate treatment strategies.
References
Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275–444.
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498.
January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104–32.
Su WW, Reddy VY, Bhasin K, Champagne J, Sangrigoli RM, Braegelmann KM, et al. Cryoballoon ablation of pulmonary veins for persistent atrial fibrillation: Results from the multicenter STOP Persistent AF trial. Heart Rhythm. 2020;17(11):1841–7.
Mansour M, Calkins H, Osorio J, Pollak SJ, Melby D, Marchlinski FE, et al. Persistent atrial fibrillation ablation with contact force-sensing catheter. JACC Clin Electrophysiol. 2020;6(8):958–69.
Hussein A, Das M, Chaturvedi V, Asfour IK, Daryanani N, Morgan M, et al. Prospective use of Ablation Index targets improves clinical outcomes following ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2017;28(9):1037–47.
Hussein A, Das M, Riva S, Morgan M, Ronayne C, Sahni A, et al. Use of Ablation Index-guided ablation results in high rates of durable pulmonary vein isolation and freedom from arrhythmia in persistent atrial fibrillation patients: the PRAISE study results. Circ Arrhythm Electrophysiol. 2018;11(9):e006576.
Solimene F, Schillaci V, Shopova G, Urraro F, Arestia A, Iuliano A, et al. Safety and efficacy of atrial fibrillation ablation guided by Ablation Index module. J Interv Card Electrophysiol. 2019;54(1):9–15.
Ioannou A, Papageorgiou N, Lim WY, Wongwarawipat T, Hunter RJ, Dhillon G, et al. Efficacy and safety of ablation index-guided catheter ablation for atrial fibrillation: an updated meta-analysis. Europace. 2020. https://doi.org/10.1093/europace/euaa224.
Thom H, Jugl SM, Palaka E, Jawla S. Matching adjusted indirect comparisons to assess comparative effectiveness of therapies: usage in scientific literature and health technology appraisals. Value Health. 2016;19(3):A100–1.
Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018;38(2):200–11.
Stabile G, Lepillier A, De Ruvo E, Scaglione M, Anselmino M, Sebag F, et al. Reproducibility of pulmonary vein isolation guided by the ablation index: 1-year outcome of the AIR registry. J Cardiovasc Electrophysiol. 2020;31(7):1694–701.
Hussein A, Gupta D, De Potter T, Spin P, Eaton K, Goldstein L, et al. Treatment of atrial fibrillation using ablation index-guided contact force ablation: a matching-adjusted indirect comparison to cryoballoon ablation. Adv Ther. 2020;37(2):785–99.
Guyot P, Ades AE, Ouwens MJNM, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol. 2012;12(1):9.
Austin PC. A tutorial on multilevel survival analysis: methods, models and applications. Int Stat Rev. 2017;85(2):185–203.
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Reporting, Vienna.
Cameron C, Varu A, Disher T, Hutton B. PRM258—methodological approaches for conducting matching-adjusted indirect comparisons involving multiple randomized controlled trials. Value Health. 2018;21:S400.
Boveda S, Metzner A, Nguyen DQ, Chun KRJ, Goehl K, Noelker G, et al. Single-procedure outcomes and quality-of-life improvement 12 months post-cryoballoon ablation in persistent atrial fibrillation: results from the multicenter CRYO4PERSISTENT AF trial. JACC Clin Electrophysiol. 2018;4(11):1440–7.
Gramlich M, Maleck C, Marquardt J, Duckheim M, Stimpfle F, Heinzmann D, et al. Cryoballoon ablation for persistent atrial fibrillation in patients without left atrial fibrosis. J Cardiovasc Electrophysiol. 2019;30(7):999–1004.
Ciconte G, Ottaviano L, de Asmundis C, Baltogiannis G, Conte G, Sieira J, et al. Pulmonary vein isolation as index procedure for persistent atrial fibrillation: one-year clinical outcome after ablation using the second-generation cryoballoon. Heart Rhythm. 2015;12(1):60–6.
Fortuni F, Casula M, Sanzo A, Angelini F, Cornara S, Somaschini A, et al. Meta-analysis comparing cryoballoon versus radiofrequency as first ablation procedure for atrial fibrillation. Am J Cardiol. 2020;125(8):1170–9.
Ravi V, Poudyal A, Pulipati P, Larsen T, Krishnan K, Trohman RG, et al. A systematic review and meta-analysis comparing second-generation cryoballoon and contact force radiofrequency ablation for initial ablation of paroxysmal and persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2020;31(10):2559–71.
Liu XH, Gao XF, Jin CL, Chen CF, Chen B, Xu YZ. Cryoballoon versus radiofrequency ablation for persistent atrial fibrillation: a systematic review and meta-analysis. Kardiol Pol. 2020;78(1):20–9.
Rattanakosit T, Franke K, Munawar DA, Page AJ, Boyd MA, Lau DH, et al. Role of indices incorporating power, force and time in AF ablation: a systematic review of literature. Heart Lung Circ. 2021. https://doi.org/10.1016/j.hlc.2021.04.007.
Romero J, Di Biase L, Mohanty S, Trivedi C, Patel K, Parides M, et al. Long-term outcomes of left atrial appendage electrical isolation in patients with nonparoxysmal atrial fibrillation: a propensity score-matched analysis. Circ Arrhythm Electrophysiol. 2020;13(11): e008390.
Romero J, Gabr M, Patel K, Briceno D, Diaz JC, Alviz I, et al. Efficacy and safety of left atrial appendage electrical isolation during catheter ablation of atrial fibrillation: an updated meta-analysis. Europace. 2021;23(2):226–37.
Romero J, Michaud GF, Avendano R, Briceño DF, Kumar S, Carlos Diaz J, et al. Benefit of left atrial appendage electrical isolation for persistent and long-standing persistent atrial fibrillation: a systematic review and meta-analysis. Europace. 2018;20(8):1268–78.
Kircher S, Arya A, Altmann D, Rolf S, Bollmann A, Sommer P, et al. Individually tailored vs. standardized substrate modification during radiofrequency catheter ablation for atrial fibrillation: a randomized study. Europace. 2018;20:1766–75.
Della Rocca DG, Tarantino N, Trivedi C, Mohanty S, Anannab A, Salwan AS, et al. Non-pulmonary vein triggers in nonparoxysmal atrial fibrillation: Implications of pathophysiology for catheter ablation. J Cardiovasc Electrophysiol. 2020;31:2154–67.
Akkaya E, Berkowitsch A, Rieth A, Erkapic D, Hamm CW, Neumann T, et al. Clinical outcome and left atrial function after left atrial roof ablation using the cryoballoon technique in patients with symptomatic persistent atrial fibrillation. Int J Cardiol. 2019;292:112–8.
Nanbu T, Yotsukura A, Suzuki G, Ishidoya Y, Sano F, Yoshida I, et al. Important factors in left atrial posterior wall isolation using 28-mm cryoballoon ablation for persistent atrial fibrillation—block line or isolation area? J Cardiovasc Electrophysiol. 2020;31(1):119–27.
Nishimura T, Yamauchi Y, Aoyagi H, Tsuchiya Y, Shigeta T, Nakamura R, et al. The clinical impact of the left atrial posterior wall lesion formation by the cryoballoon application for persistent atrial fibrillation: feasibility and clinical implications. J Cardiovasc Electrophysiol. 2019;30(6):805–14.
Zei PC, Hunter TD, Gache LM, O’Riordan G, Baykaner T, Brodt CR. Low-fluoroscopy atrial fibrillation ablation with contact force and ultrasound technologies: a learning curve. Pragmat Observ Res. 2019;10:1–7.
Acknowledgements
Funding
This study (including Rapid Service and Open Access Fees) was funded by Biosense Webster, Inc., Irvine, California, USA.
Medical Writing, Editorial and Other Assistance
Authors would like to thank Teresa Kangappaden, Hons BMath, Meghan Hughes, PhD, and Coby Martin, MSc, who were all with CRG-EVERSANA (formerly Cornerstone Research Group, Inc.) at the time of support, for systematic literature review support. This support was funded by Biosense Webster, Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
LG, TW, and MV contributed to the conception and design of the research and provided critical revisions and intellectual content to the manuscript. GS, AH, and DG provided substantial intellectual content and revisions to the manuscript. PS, LP, and KD conducted analyses, discussed the results, drafted the manuscript, and contributed to the final version of the manuscript. All authors provided critical and intellectual feedback of the research, analysis, and manuscript.
Disclosures
Laura Goldstein is an employee of Biosense Webster, Inc. Tom Wei is an employee of Biosense Webster, Inc. Maria Velleca is an employee of Johnson & Johnson Medical S.p.A. Giuseppe Stabile is a consultant to Biosense Webster, Inc. Dhiraj Gupta is a consultant to Biosense Webster, Inc. Ahmed Hussein is a consultant to Biosense Webster, Inc. Paul Spin is an employee of EVERSANA. Kaitlyn Dawkins is an employee of EVERSANA. Leena Patel is an employee of EVERSANA. EVERSANA is a consultant to pharmaceutical and medical device companies, including Biosense Webster, Inc.
Compliance with Ethical Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hussein, A., Stabile, G., Dawkins, K. et al. Effectiveness of Radiofrequency Catheter Ablation Using Ablation Index Versus Second Generation Cryoballoon in the Treatment of Persistent Atrial Fibrillation: A Matching-Adjusted Indirect Comparison. Adv Ther 38, 4388–4402 (2021). https://doi.org/10.1007/s12325-021-01846-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01846-z