Abstract
Introduction
In the EDITION clinical trial programme, patients with type 2 diabetes mellitus (T2DM) receiving insulin glargine (IGlar) U300 required 10–15% more insulin than those receiving IGlar U100. This study sought to determine whether this difference was apparent in real-world practice.
Methods
In this observational, retrospective cohort study, electronic medical records in the Big-Pac® database (Real Life Data) relating to adult insulin-naïve patients with T2DM who initiated IGlar U100 or U300 treatment in Spain in 2016–2017 and remained on treatment for 18 months were selected. IGlar U100- and U300-treated patients were matched 1:1 (propensity score matching). The primary analysis compared changes from baseline in mean daily IGlar dose (U and U/kg) at 6 (± 2), 12 (± 2) and 18 (± 2) months between cohorts (paired t tests). Changes in glycated haemoglobin (HbA1c) and weight were analysed descriptively.
Results
The IGlar U100 and U300 cohorts included 556 matched pairs (46.9% female) with the following mean (standard deviation) values at baseline, respectively: age 63.6 (12.8) versus 63.7 (11.9) years; years since diagnosis 9.5 (1.4) versus 9.5 (1.3); HbA1c 8.8 (1.3) versus 8.7 (1.5) %; weight 84.6 (16.9) versus 84.7 (17.1) kg. Mean IGlar dose at baseline was 0.19 U/kg/day (both cohorts). Patients receiving IGlar U300 showed a greater increase from baseline in IGlar dose at 6, 12 and 18 months [mean dose (U/kg/day) 5.1%, 10.3% and 12.8% greater, respectively, in IGlar U300-treated patients]. Mean HbA1c was 8.1% in both cohorts at 18 months. Mean (SD) weight at 18 months with IGlar U100 and IGlar300 was 86.8 (17.0) kg and 85.0 (17.1) kg, respectively.
Conclusion
In real-world practice, insulin dose was significantly higher in IGlar U300-treated than U100-treated patients at 6, 12 and 18 months, with similar reductions in HbA1c. At equal IGlar price/unit in Spain, the increased dose requirements of IGlar U300 would result in higher costs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
In clinical trials, patients with type 2 diabetes mellitus (T2DM) receiving insulin glargine (IGlar) U300 required 10–15% more insulin than those receiving IGlar U100 |
There is limited real-world evidence about the doses of these two IGlar preparations |
The real-world DosInGlar study aimed to determine the doses of insulin for IGlar U300 versus IGlar U100 in insulin-naïve patients with T2DM in the 18 months after treatment initiation |
What was learned from this study? |
The results showed that insulin doses over time are higher in patients receiving IGlar U300 versus IGlar U100 in the real-world setting, although glycaemic control is similar for both preparations |
When initiating insulin treatment, in addition to an antidiabetic effect, risk of hypoglycaemia and effect on body weight, physicians should assess the possible cost implications associated with total dose, which will potentially be higher with IGlar U300 |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14535843.
Introduction
Type 2 diabetes mellitus (T2DM) is a progressive disease that requires treatment intensification to maintain glycaemic control over time [1]. As a result, many patients eventually require insulin [2]. When insulin therapy is first introduced into the antidiabetic regimen of a patient with T2DM, basal insulin is usually the first option [3]. Insulin glargine (IGlar) was the first once-daily, long-acting insulin analogue to be introduced into clinical practice [4]. At this time, it was formulated as a 100 unit/ml preparation [IGlar U100 (Lantus®)] [4]. IGlar U100 has demonstrated consistent efficacy and safety in numerous randomised controlled trials [4] and real-world evidence studies [5] and represents the ‘gold standard’ against which new basal insulin analogues are assessed [6]. Since the introduction of IGlar U100, a number of 100 unit/ml insulin glargine biosimilars with similar efficacy and safety profiles have become available [7].
IGlar is also available as a 300 unit/ml formulation [IGlar U300 (Toujeo®)] [8]. IGlar U100 and IGlar U300 have different pharmacokinetic/pharmacodynamic profiles. The mean duration of action of IGlar U100 is 25.5 h under euglycaemic clamp conditions, following a single-dose of 0.3 U/kg [9]. The pharmacokinetic profiles of IGlar U100 and IGlar U300 were compared following seven 0.4 U/kg doses with estimated median terminal half-lives of 19 and 13.5 h, respectively [10]. IGlar U300 also has a flatter and more extended time-action profile than IGlar U100 [11], and this may result in more stable and sustained glycaemic control over the 24-h inter-dosing interval [11]. An additional difference between these two insulin preparations is that, for the same number of units of insulin, the volume of IGlar U300 is approximately one-third that of IGlar U100 [11].
The EDITION clinical trial programme [11] was designed to evaluate the efficacy and safety of IGlar U300 versus IGlar U100. The majority of the EDITION trials enrolled patients who had previously used insulin, but EDITION 3, which enrolled 878 insulin-naïve patients with T2DM from across North America, Europe and Japan, was an exception [11, 12]. In this trial, although the basal insulin dose increased in both groups, the mean dose was ~17% greater in the IGlar U300 group at 6 months (0.62 U/kg/day vs. 0.53 U/kg/day), with no significant difference between groups in glycaemic control and nocturnal symptomatic hypoglycaemia [12]. These disparities in insulin dose between IGlar U300 and IGlar U100 reflect the results of the EDITION programme as a whole, in which a 10–15% higher dose of IGlar U300 versus IGlar U100 was typical [11], and are acknowledged in the summaries of product characteristics for the IGlar U300 and U100 products [8, 13, 14].
The EDITION data were generated as part of a clinical trial programme. However, the importance of taking real-world data into account when making treatment decisions is gaining recognition [15,16,17], and it is for this reason that we conducted the DosInGlar study. Until now, data relating to the dosage profile of IGlar U300 vs. IGlar U100 in insulin-naïve patients with T2DM in routine clinical practice were limited to a 6-month retrospective study (REALITY), which involved 376 propensity score-matched patients [18], and a retrospective study utilising medical record data to investigate treatment dosing patterns and clinical outcomes in 390 patients, published by Gupta et al. [19]. In the REALITY population, the mean dose of IGlar U300 was ~17% higher than the mean dose of IGlar U100 at the end of follow-up, with no difference between groups in mean glycated haemoglobin (HbA1c) [18], whereas Gupta et al. [19] reported similar doses of IGlar U100 and U300 and a similar HbA1c between groups.
The primary aim of the DosInGlar study was to compare the dosage profiles of IGlar U100 and IGlar U300 when used in insulin-naïve patients with T2DM in clinical practice over a longer follow-up period than has been reported previously (18 months). In addition, we aimed to describe the sociodemographic and clinical characteristics of IGlar U100- and IGlar U300-treated patients at the time of treatment initiation and the changes in HbA1c, fasting plasma glucose (FPG), and body weight up to 18 months after treatment initiation.
Methods
Study Design and Patient Population
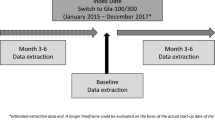
The DosInGlar study was an observational retrospective cohort study based on electronic medical records in the ENCePP-registered Big-Pac database [20] in Spain (Fig. 1). Big-Pac is a source of dissociated and anonymised medical records from 1.8 million patients attending primary care and specialised centres. The records held by Big-Pac are representative of the Spanish population in terms of prevalence of specific diseases (including diabetes) and biochemical and anthropometric patient characteristics [21]. The medical records held by Big-Pac are collected monthly from a panel of > 1200 office-based primary care physicians and 900 specialists in 7 of the 17 regions in Spain. The database includes information relating to demographic characteristics, diagnoses, morbidity and mortality data [International Classification of Diseases (ICD) codes], medication details, clinical scales, biochemistry data, anthropometric characteristics and use of resources (physician visits, hospital admissions, tests ordered). Data relating to hypoglycaemic episodes are included in the database, but mild and moderate cases may be under-reported. This study was approved by the local ethics committee (Hospital de Tarrasa in Barcelona).
The Big-Pac database was used to identify adult patients with a diagnosis of T2DM [ICD, Tenth Revision, Clinical Modifications (ICD-10-CM) code: E10–E14] who initiated treatment with IGlar U100 or IGlar U300 (identified from pharmacy claims file anatomical therapeutic chemical codes) during the period 1 January 2016 to 31 December 2017 inclusive (the inclusion period; Fig. 1). At this time, LY2963016 (Abasaglar®) was the only IGlar biosimilar available in Spain; the insulin formulations used by patients therefore comprised Toujeo® (IGlar U300), Lantus®, or Abasaglar® (both IGlar U100). The date on which each patient initiated IGlar U100 or IGlar U300 treatment (the index therapy) was designated as the index date, and patients were followed for 18 months from this date. In addition to index, the following time windows at each temporal data point were defined: 6 (± 2), 12 (± 2) or 18 (± 2) months (hereafter written as 6, 12 and 18 months).
Inclusion criteria included T2DM; age ≥ 18 years on the index date; insulin naïve for ≥ 1 year prior to the index date; received one or more prescription during the follow-up period; two or more health records included in the computer system; and remained on IGlar treatment, without discontinuation, for ≥ 18 months from the index date. Discontinuation was defined as a period of ≥ 6 months during which no insulin was dispensed. Patients were excluded from the study if they were permanently institutionalised, had previously received IGlar therapy, were receiving a glucagon-like peptide 1 receptor agonist (GLP-1 RA) or prandial insulin on the index date, had a diagnosis of type 1 or gestational diabetes, or if their record was missing two or more temporal data points for the same variable among any of HbA1c, FPG, body weight or IGlar dose.
Study Endpoints and Data Extraction
The primary study objective was to compare the change from baseline in daily dose (U and U/kg) of IGlar at 6, 12 and 18 months after the index date between the IGlar U100- and IGlar U300-treated cohorts. Dispensed medication was used for the analysis. The daily doses of IGlar U100 and U300 (U/day) were recorded for the index date (first prescription). At months 6, 12 and 18, the daily dose was calculated as the mean of the doses [U/day (provided in the record) and U/kg/day (calculated)] for all dispensed prescriptions within the relevant ± 2-month interval. The secondary study objectives were to describe the sociodemographic and clinical characteristics at index and the changes in HbA1c, FPG and body weight at 6, 12 and 18 months in the two cohorts. Comorbidity burden was assessed at index by the Charlson Comorbidity Index [22]. The HbA1c, FPG and body weight values used were those obtained within 6 (± 2), 12 (± 2) and 18 (± 2) months after the index date.
Statistical Analysis
To minimize selection bias, propensity score matching (PSM) was used to match each patient who initiated IGlar U100 therapy 1:1 with a patient who initiated IGlar U300. Baseline covariates used in the PSM multivariate regression model included age, sex, time from diagnosis, macrovascular complications, microvascular complications, antidiabetic medication, concomitant medication, HbA1c, body weight, and IGlar dose at index. Patients were matched using the greedy nearest neighbour method without replacement, with a calliper of 0.2 standard deviations (SDs).
Based on an IGlar daily dose SD of 25 units [23], a sample size of 500 per cohort would allow detection of a difference in glargine dose of ≥ 5.1 U/day (i.e. 0.06 U/kg/day for an 85 kg individual) with 90% power (two-sided two-sample t test with a type I error rate of 0.05). These calculations did not account for matching between samples and subsequent reduction in variation of the outcome difference between cohorts and were therefore conservative. The 1:1 matching that was inherent in the PSM procedure reduced the effective sample size to that of the smaller of the two cohorts.
Changes from baseline in IGlar daily dose (U and U/kg) at 6, 12 and 18 months were compared between the matched cohorts using paired t tests. A hierarchical testing strategy was used (i.e. the difference between the two cohorts in dose change from baseline was tested sequentially in the following order: month 18, month 12, month 6). Subsequent time points were only tested if all previous time points had shown a statistically significant difference. This ensured overall control of the type I error. Secondary objectives (changes in HbA1c, FPG and body weight at 6, 12 and 18 months) were analysed descriptively. Absolute values of IGlar dose, HbA1c, FPG and body weight at each time point were also computed. Qualitative data were expressed as absolute and relative frequencies and quantitative data as mean, SD and 95% confidence intervals (CIs). A p value < 0.05 signified statistical significance.
All analyses were performed using SPSSWIN v23 (IBM SPSS Statistics: IBM, Armonk, NY, USA).
Results
From a total database patient population of 49,317 who were aged ≥ 18 years with a diagnosis of T2DM, 2492 initiated IGlar U100 and 1297 initiated IGlar U300 during the inclusion period. Approximately 2% of each group were lost to follow-up, and 24% in each group discontinued index therapy before 18 months (Fig. 2). After exclusions for missing data, loss to follow-up, discontinuation of the index therapy after < 18 months, and use of a GLP-1 RA or prandial insulin on the index date, the IGlar U100 and IGlar U300 cohorts included 1109 and 559 patients, respectively. This was reduced to 556 patients in each cohort after subsequent PSM (Fig. 2).
Patient flowchart. *Baseline covariates included age, sex, time from diagnosis, HbA1c, weight, IGlar dose, macrovascular complications, microvascular complications, antidiabetic medication and concomitant medication. Method: 1:1 PSM; greedy nearest neighbour without replacement. GLP-1 RA glucagon-like peptide-1 receptor agonist; HbA1c glycated haemoglobin; IGlar insulin glargine; PSM propensity score matching; T2DM type 2 diabetes mellitus
The baseline sociodemographic characteristics of the two cohorts were similar prior to [Table S1 in the electronic supplementary material (ESM)] and after (Table 1) matching, with similar values in the IGlar U100 and IGlar U300 matched cohorts for percentage of female patients (46.9% for both), mean age (63.6 and 63.7 years), time since diagnosis of T2DM (9.5 years for both), index HbA1c (8.8 and 8.7%), index body weight (84.6 and 84.7 kg), index FPG (10.8 and 10.9 mmol/l) and Charlson Comorbidity Index score (2.1 and 2.2). The percentages of each cohort receiving each of a range of concomitant antidiabetic medications were also similar (Table 1).
At index, the mean daily dose of IGlar was 0.19 U/kg in both cohorts (Table 2) (absolute mean daily doses: IGlar U100, 16.3 U; IGlar U300, 16.1 U). The mean change from baseline in IGlar dose (U/kg/day) was significantly higher in the IGlar U300-treated patients than in those receiving IGlar U100 at 6, 12 and 18 months, with differences of 0.02, 0.04 and 0.05 U/kg/day, respectively (p ≤ 0.001 for all; mean doses of 0.44 vs. 0.39 U/kg/day and 21.1 vs. 17.2 U/day at 18 months). This represents 5.1%, 10.3% and 12.8% higher doses of IGlar U300 vs. IGlar U100 at these time points (Fig. 3).
Mean HbA1c decreased by similar amounts in both groups at 6, 12 and 18 months, achieving mean values of 8.1% and 8.1% in the IGlar U100 and IGlar U300 groups, respectively, at 18 months (Table 2; Fig. 4). Mean FPG also decreased by similar amounts in the two groups (IGlar U100 vs. IGlar U300: 6 months, − 0.4 vs. − 0.4 mmol/l; 12 months, − 0.4 vs. − 0.6 mmol/l; 18 months, − 0.6 vs. − 0.8 mmol/l) (Fig. 4), whereas mean body weight—which was similar between groups at baseline—increased more in the IGlar U100 group than in those receiving IGlar U300 at 6, 12 and 18 months, culminating in mean increases of 2.1 kg (IGlar U100) and 0.3 kg (IGlar U300) at 18 months [between-group difference, IGlar U100 vs. IGlar U300: 1.87 kg (95% CI 1.42–2.31)]. Mean (SD) weight at 18 months with IGlar U100 and IGlar300 was 86.8 (17.0) kg and 85.0 (17.1) kg, respectively.
Mean change from baseline in HbA1c (a), mean FPG (b) and HbA1c (c) at 6, 12 and 18 months in patients treated with IGlar U300 or IGlar U100. FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; IGlar insulin glargine; m months; SD standard deviation. Values in graphs represent mean ± 95% confidence intervals
Discussion
The DosInGlar study suggests that, in real-world clinical practice, similar mean HbA1c and FPG values were achieved using 13% higher doses of IGlar U300 versus IGlar U100 at 18 months after treatment initiation in insulin-naïve patients with T2DM. Our results therefore support the current American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) consensus report for the management of hyperglycaemia in T2DM and the European summary of product characteristics for Toujeo® (IGlar U300), which state that a 10–14% [3] or 10–18% [8] higher dose of IGlar U300 versus IGlar U100 may be required to achieve similar glycaemic targets. Our results are also aligned with the results of randomised clinical trials and other real-world studies that have suggested that higher doses of IGlar U300 than IGlar U100 are needed to achieve a particular level of glycaemic control [11, 18, 24,25,26]. In many of these studies, as in the DosInGlar study, these patients with T2DM were insulin naïve when IGlar therapy was initiated [18, 24, 25]. While the reason for the dose difference between IGlar U300 and IGlar U100 is currently unknown, it may be due to IGlar U300 spending more time in the subcutaneous depot than IGlar U100, thereby allowing greater enzymatic inactivation of the glargine molecule [25].
The difference in dosing profile between IGlar U100 and IGlar U300 has a number of implications for both patients and healthcare systems. Based on the findings of the DosInGlar study, in countries where IGlar U100 and IGlar U300 have the same (or similar) cost per unit (e.g. Spain [27]), the higher dose requirements of IGlar U300 to achieve a similar glycaemic control would result in higher treatment costs (13% more expensive, on a unit/kg basis, if the price per unit is equal). Differences among countries in healthcare systems, insulin prescribing and utilisation patterns, etc., may need to be considered before these results can be extrapolated to other countries. A further implication is that it may take longer to reach the optimal dose of insulin with IGlar U300 than with IGlar U100. This is supported by the results of the EDITION clinical trial programme [11] and by our data, which show that the mean daily dose of IGlar U100 remained unchanged between 6 and 18 months, but the dose of IGlar U300 still had not plateaued 18 months after treatment initiation (Fig. 3).
Although hypoglycaemia data are not available for the DosInGlar study, this topic merits discussion because it is an important consideration when determining which insulin preparation should be prescribed for a patient. Although the current ADA/EASD consensus report states that IGlar U300 is associated with a lower risk of nocturnal hypoglycaemia than IGlar U100 [3], this is not conclusively borne out by clinical trial results or real-world data involving insulin-naïve patients with T2DM. For example, the annualised rate of hypoglycaemia was not significantly lower for IGlar U300 versus IGlar U100 in any of the eight categories of hypoglycaemia examined in EDITION AP [24] and in only one of the eight categories (documented symptomatic hypoglycaemia: ≤ 3.9 mmol/l over 24 h) in EDITION 3 [12]. Moreover, in EDITION 3, the percentage of participants experiencing the main secondary efficacy endpoint (one or more nocturnal confirmed or severe hypoglycaemic event) was 16% with IGlar U300 and 17% with IGlar U100 [relative risk 0.89 (95% CI 0.66–1.20)] [12], and in the real-world REALITY study, similar on-study percentage increases were seen in the proportions of IGlar U100- and IGlar U300-treated patients who reported at least one incidence of hypoglycaemia per week (IGlar U100: 5.5% at baseline, 11.0% at follow-up; IGlar U300: 3.3% at baseline, 8.7% at follow-up) [18].
Given the potential for weight gain in insulin-treated patients [3], body weight is another important consideration, and we should again look to clinical trial and real-world data involving insulin-naïve patients with T2DM for insight. Other studies have consistently shown similar mean on-study changes in body weight in IGlar U100- and IGlar U300-treated patients with T2DM, with no significant differences between treatments in either clinical trials or real-world studies [18, 24, 25] and some studies reporting lower weight gain in the IGlar U100 group than the IGlar U300 group [18, 24]. However, in the DosInGlar study, mean body weight increased to a greater extent in the IGlar U100-treated group than in those receiving IGlar U300. An influence of concomitant medication is unlikely to underlie this finding, as no apparent differences in background antidiabetic therapy between groups were found. Weight gain and hypoglycaemia often co-present as unwanted effects of insulin treatment in patients with T2DM [28], and there is some evidence for an association between weight gain and hypoglycaemia from the Diabetes Control and Complications Trial (DCCT) [29]. Therefore, it could be hypothesised that the increased weight gain in the IGlar U100 group reflects a relative increase in hypoglycaemic events compared with the IGlar U300 group. Hypoglycaemia data were not available for the DosInGlar study, although it has been evaluated in other studies of insulin-naïve T2DM patients that compared IGlar U100 and IGlar U300. The 6-month REALITY study (18) showed that insulin-naïve patients previously treated with IGlar U100 had higher rates of hypoglycaemia relative to those treated with IGlar U300, although this was not accompanied by additional weight gain. Moreover, data from the EDITION-3 trial show that annualised rates of nocturnal confirmed (≤ 3.9 mmol/l) or severe hypoglycaemic events were similar for IGlar U100 and IGlar U300 at 6 and 12 months [12, 25], with a slight (not significant) increase in weight gain in the IGlar U100 group at 6 and 12 months. It seems difficult to consolidate these previous data with the possibility that increased weight gain with IGlar U100 in the DosInGlar study was related to hypoglycaemia (although it cannot be excluded). Alternatively, other factors such as lifestyle interventions and socio-economic status, for which data were also not collected, could have influenced this parameter. In addition, it is important to note that body weight was a descriptive endpoint and that the study was not designed to test differences between cohorts in this outcome. These data should therefore be interpreted with caution.
It is generally accepted that similar levels of glycaemic control are achieved with IGlar U100 and IGlar U300 [11], and this is supported by the results of the DosInGlar study. However, it is notable that the mean HbA1c at 18 months after treatment initiation (8.1% in both treatment groups) was higher than the goal of 7.0% that is typically recommended for most patients [3]. As already noted, the IGlar U300 dose had not plateaued 18 months after treatment initiation, and we therefore cannot exclude the possibility of a further reduction in HbA1c for IGlar U300 if follow-up had been longer. However, the incline of the slope between 12 and 18 months is slight (Fig. 3), which suggests that any further dose increase would have been minimal (potential for additional reduction in HbA1c is therefore predicted to be negligible; moreover, robust analysis of any resulting difference would not be possible because of the lack of statistical power to detect such small dose differences). The suboptimal HbA1c values at 18 months indicate that whichever IGlar formulation patients with T2DM receive in real-world clinical practice, additional lifestyle and pharmacological interventions and/or intensification of insulin therapy are frequently needed. Lifestyle interventions form the cornerstone of effective diabetes management, and clinical guidelines place an emphasis on both lifestyle management and patient education [3]. Therapeutic inertia represents an additional problem in patients with T2DM [30]. Nevertheless, adherence to the detailed guidance provided by current guidelines on active management [3] should increase the likelihood of treatment goals being achieved.
There were some differences in the baseline sociodemographic characteristics of this study population versus the randomised EDITION-3 [12] multicentre trial that also compared IGlar U100 and U300 in an insulin-naïve population with type 2 diabetes; patients in this real-world study were older and had a lower body weight than those in the EDITION-3 study (time from diagnosis and HbA1c were similar between these studies). The strict inclusion and exclusion criteria of randomised controlled trials may lead to discrepancies in terms of age, sex, comorbidities, disease severity and comedication [31, 32]. Patients in controlled trials may also exhibit different behaviours, including higher medication adherence and increased tolerance of adverse events [31]. Compared to the real-world REALITY study, in a Canadian population, patients in our study were older, had shorter times from diagnosis and lower HbA1c—this supports an influence of region on sociodemographic and disease characteristics of populations with T2DM in real-world settings (i.e. a Spanish population versus a more diverse population distributed across several countries). The potential for regional differences in population characteristics should therefore be considered when comparing different studies.
This study was subject to a number of limitations. The retrospective, observational nature of the study and the fact that it was based on electronic medical records mean that some data may not have been recorded. Moreover, the data available were limited (e.g. there were no data relating to time of IGlar administration or adherence, and the hypoglycaemic event data were not assessed as they were only partially available because of under-reporting). The analysis did not account for individual antidiabetic therapies used as part of ‘oral combination therapy’. As a result, the percentages of patients recorded as using metformin may be artificially low. The implication of increased cost for IGlar U300 (because of the higher dose needed to achieve a similar antidiabetic effect to U100) is applicable where the price per unit of insulin is similar or equal (e.g. Spain [27], at time of publication), but this may not necessarily be the case in other regions or may be subject to future change. Additional limitations include the requirement for patients to be on insulin for 18 months. This means that our findings cannot be generalised to all IGlar-treated patients with T2DM. It is also possible that our findings may be affected by confounding factors (e.g. level of education and socio-economic status), which may affect compliance with lifestyle and medication recommendations [33,34,35,36] and for which data were not available, and by selection bias. The potential for the latter was minimised, however, by the use of PSM. The use of descriptive analyses only for the secondary objectives represents a further limitation.
The potential for real-world evidence to complement clinical trials data is increasingly being recognised [37], and the confirmation of clinical trial data provided by the DosInGlar study is important. We have shown that, in insulin-naïve patients with T2DM who initiate insulin therapy, use of IGlar U300 is associated with higher doses of insulin than IGlar U100 in real-world clinical practice. Moreover, the degree of glycaemic control achieved was similar with the two preparations. The findings of this real-world study are supported by those of the EDITION 3 and EDITION AP clinical trials, which have also shown that, in previously insulin-naïve patients with T2DM, the dose of IGlar U300 associated with a particular level of glycaemic control is higher than the dose of IGlar U100. Taking into account the similarities between IGlar U300 and IGlar U100 in terms of the baseline sociodemographic characteristics and disease burden (Charlson Comorbidity Index) of patients with T2DM who initiate therapy, an antidiabetic effect (reduction in HbA1c and FPG), risk of hypoglycaemia and effect on body weight, physicians should assess the possible cost implications associated with total dose—which will potentially be higher with IGlar U300—when initiating insulin treatment.
References
Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S151–6. https://doi.org/10.2337/dc09-S301.
Inzucchi SE, Bergenstah RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–9. https://doi.org/10.2337/dc14-2441.
Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–701. https://doi.org/10.2337/dci18-0033.
Hilgenfeld R, Seipke G, Berchtold H, Owens DR. The evolution of insulin glargine and its continuing contribution to diabetes care. Drugs. 2014;74(8):911–27. https://doi.org/10.1007/s40265-014-0226-4.
Hirose T, Chen CC, Ahn KJ, Kiljański J. Use of insulin glargine 100 U/mL for the treatment of type 2 diabetes mellitus in East Asians: a review. Diabetes Ther. 2019;10(3):805–33. https://doi.org/10.1007/s13300-019-0613-7.
Standl E, Owen DR. New long-acting basal insulins: does benefit outweigh cost? Diabetes Care. 2016;39(Suppl 2):S172–9.
Davies M, Dahl D, Heise T, Kiljanski J, Mathieu C. Introduction of biosimilar insulins in Europe. Diabet Med. 2017;34(10):1340–53. https://doi.org/10.1111/dme.13400.
Sanofi-Aventis Deutschland GmbH. Toujeo® Summary of Product Characteristics. 2020. https://www.ema.europa.eu/documents/product-information/toujeo-epar-product-information_en.pdf. Accessed 5 Oct 2020.
Linnebjerg H, Lam ECQ, Zhang X, et al. Duration of action of two insulin glargine products, LY2963016 insulin glargine and Lantus insulin glargine, in subjects with type 1 diabetes mellitus. Diabetes Obes Metab. 2017;19(1):33–9. https://doi.org/10.1111/dom.12759.
Becker RHA, Dahmen R, Bergmann K, et al. New insulin glargine 300 units∙mL-1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 units∙mL-1. Diabetes Care. 2015;38(4):637–43. https://doi.org/10.2337/dc14-0006.
Vargas-Uricoechea H. Efficacy and safety of insulin glargine 300 U/mL versus 100 U/mL in diabetes mellitus: a comprehensive review of the literature. J Diabetes Res. 2018;2018:2052101. https://doi.org/10.1155/2018/2052101.
Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17(4):386–94. https://doi.org/10.1111/dom.12438.
Eli Lilly Nederland B.V. Abasaglar summary of product characteristics. 2020. https://www.ema.europa.eu/en/documents/product-information/abasaglar-previously-abasria-epar-product-information_en.pdf. Accessed 5 Oct 2020.
Sanofi-Aventis Deutschland GmbH. Lantus® summary of product characteristics. 2015. https://www.ema.europa.eu/en/documents/product-information/lantus-epar-product-information_en.pdf. Accessed 27 Oct 2020.
United States Food and Drug Administration. Real-world evidence. 2020. https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence. Accessed 13 July 2020.
Katkade VB, Sanders KN, Zou KH. Real world data: an opportunity to supplement existing evidence for the use of long-established medicines in health care decision making. J Multidiscip Healthc. 2018;11:295–304. https://doi.org/10.2147/JMDH.S160029.
Zaccardi F, Davies MJ, Khunti K. The present and future scope of real-world evidence research in diabetes: What questions can and cannot be answered and what might be possible in the future? Diabetes Obes Metab. 2020;22(Suppl 3):21–34. https://doi.org/10.1111/dom.13929.
Abitbol A, Brown RE, Jiandani D, Sauriol L, Aronson R. Real-world health outcomes of insulin glargine 300 U/ml vs insulin glargine 100 U/ml in adults with type 1 and type 2 diabetes in the Canadian LMC Diabetes Patient Registry: The REALITY study. Can J Diabetes. 2019;43(7):504-509.e1. https://doi.org/10.1016/j.jcjd.2019.04.012.
Gupta S, Wang H, Skolnik N, et al. Treatment dosing patterns and clinical outcomes for patients with type 2 diabetes starting or switching to treatment with insulin glargine (300 units per milliliter) in a real-world setting: a retrospective observational study. Adv Ther. 2018;35(1):43–55. https://doi.org/10.1007/s12325-017-0651-3.
European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. 2019. http://www.encepp.eu/encepp/viewResource.htm?id=29236. Accessed 8 Jun 2020.
Sicras-Mainar A, Enriquez JL, Hernández I, Sicras-Navarro A. Validation and representativeness of the Spanish BIG-PAC database: Integrated computerized medical records for research into epidemiology, medicines and health resource use (real word evidence). Value Health. 2019;22(3):S734.
Austin SR, Wong YN, Uzzo RG, et al. Why summary comorbidity measures such as the Charlson Comorbidity Index and Elixhauser score work. Med Care. 2015;53(9):e65–72. https://doi.org/10.1097/MLR.0b013e318297429c.
Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD-2). Diabetes Care. 2015;38(12):2241–9. https://doi.org/10.2337/dc14-1625.
Ji L, Kang ES, Dong X, et al. Efficacy and safety of insulin glargine 300 U/mL versus insulin glargine 100 U/mL in Asia Pacific insulin-naïve people with type 2 diabetes: The EDITION AP randomized controlled trial. Diabetes Obes Metab. 2020;22(4):612–21. https://doi.org/10.1111/dom.13936.
Bolli GB, Riddle MC, Bergenstal RM, et al. Glycaemic control and hypoglycaemia with insulin glargine 300U/mL versus insulin glargine 100U/mL in insulin-naïve people with type 2 diabetes: 12-month results from the EDITION 3 trial. Diabetes Metab. 2017;43(4):351–8. https://doi.org/10.1016/j.diabet.2017.04.007.
Ritzel R, Roussel R, Giaccari A, Vora J, Brulle-Wohlhueter C, Yki-Järvinen H. Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes. Diabetes Obes Metab. 2018;20(3):541–8. https://doi.org/10.1111/dom.13105.
Base de Datos de medicamentos del Consejo General de Farmacéuticos (Bot PLUS 2.0). 2020. https://botplusweb.portalfarma.com. Accessed 10 June 2020.
DeVries JH, Desouza C, Bellary S, et al. Achieving glycaemic control without weight gain, hypoglycaemia, or gastrointestinal adverse events in type 2 diabetes in the SUSTAIN clinical trial programme. Diabetes Obes Metab. 2018;20(10):2426–34. https://doi.org/10.1111/dom.13396.
Purnell JQ, Dev RK, Steffes MW, et al. Relationship of family history of type 2 diabetes, hypoglycemia, and autoantibodies to weight gain and lipids with intensive and conventional therapy in the Diabetes Control and Complications Trial. Diabetes. 2003;52(10):2623–9. https://doi.org/10.2337/diabetes.52.10.2623.
Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20(2):427–37. https://doi.org/10.1111/dom.13088.
Munk NE, Knudsen JS, Pottegård A, et al. Differences between randomized clinical trial participants and real-world empagliflozin users and the changes in their glycated hemoglobin levels. JAMA Netw Open. 2020;3(2):e1920949. https://doi.org/10.1001/jamanetworkopen.2019.20949.
Monti S, Grosso B, Todoerti M, Caporali R. Randomized controlled trials and real-world data: differences and similarities to untangle literature data. Rheumatology (Oxford). 2018;57(57 Suppl 7):54–8. https://doi.org/10.1093/rheumatology/key109.
Houle J, Lauzier-Jobin F, Beaulieu MD, et al. Socioeconomic status and glycemic control in adult patients with type 2 diabetes: a mediation analysis. BMJ Open Diabetes Res Care. 2016;4(1):e000184. https://doi.org/10.1136/bmjdrc-2015-000184.
Rahman M, Nakamura K, Hasan SMM, Seino K, Mostofa G. Mediators of the association between low socioeconomic status and poor glycemic control among type 2 diabetics in Bangladesh. Sci Rep. 2020;10(1):6690. https://doi.org/10.1038/s41598-020-63253-8.
Parajuli J, Saleh F, Thapa N, Ali L. Factors associated with nonadherence to diet and physical activity among Nepalese type 2 diabetes patients; a cross sectional study. BMC Res Notes. 2014;7:758. https://doi.org/10.1186/1756-0500-7-758.
Kirkman MS, Rowan-Martin MT, Levin R, et al. Determinants of adherence to diabetes medications: findings from a large pharmacy claims database. Diabetes Care. 2015;38(4):604–9. https://doi.org/10.2337/dc14-2098.
Bartlett VL, Dhruva SS, Shah ND, Ryan P, Ross JS. Feasibility of using real-world data to replicate clinical trial evidence. JAMA Netw Open. 2019;2(10):e1912869. https://doi.org/10.1001/jamanetworkopen.2019.1286.
Acknowledgements
Funding
This study was sponsored by Eli Lilly and Company, Indianapolis, IN, USA, who also funded the rapid publication of this article.
Medical Writing Assistance
The authors would like to acknowledge Janet Douglas and Sheridan Henness (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this article. Support for this assistance was funded by Eli Lilly and Company, Indianapolis, IN, USA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Miriam Rubio was involved in the analysis of the data, interpretation of the data, drafting of the manuscript and critical revision of the manuscript for important intellectual content. Irene Romera was involved in the conception and design of the study, interpretation of the data and critical revision of the manuscript for important intellectual content. Natalia Duque was involved in the interpretation of the data for the study and critical revision of the manuscript for important intellectual content. Esther Artime was involved in the conception and design of the study, analysis of the data, interpretation of the data and critical revision of the manuscript for important intellectual content. Silvia Díaz was involved in the conception and design of the study, interpretation of the data and critical revision of the manuscript for important intellectual content. Jesús Reviriego was involved in the conception and design of the study, acquisition and analysis of the data, interpretation of the data and critical revision of the manuscript for important intellectual content. Xavier Mundet was involved in the interpretation of the data for the study and critical revision of the manuscript for important intellectual content. Juan J. Gorgojo-Martinez was involved in the interpretation of the data for the study, drafting of the manuscript and critical revision of the manuscript for important intellectual content. Jeremie Lebrec was involved in the analysis of the data, interpretation of the data and critical revision of the manuscript for important intellectual content. Enrique Carretero-Anibarro was involved in the analysis of the data, interpretation of the data and critical revision of the manuscript for important intellectual content. Antoni Sicras-Mainar was involved in the conception and design of the study, acquisition and analysis of the data, interpretation of the data and critical revision of the manuscript for important intellectual content.
Prior Presentation
The data presented in this paper have been previously presented (Artime E et al. Dosage profile of insulin glargine U100 and U300 in patients with type 2 diabetes mellitus in real clinical practice: DosInGlar Study. XXXI Congress of the Spanish Society of Diabetes, 21–23 June 2020; Duque N et al. Dosage profile of insulin glargine U100 and U300 in patients with type 2 diabetes mellitus in real clinical practice: DosInGlar Study. European Association for the Study of Diabetes 56th annual meeting, 21–25 September 2020).
Disclosures
Irene Romera, Natalia Duque, Silvia Díaz and Miriam Rubio are full-time employees of Lilly Spain. Jeremie Lebrec is a consultant for Eli Lilly and Company. Miriam Rubio reports being a minor shareholder in Eli Lilly. Irene Romera, Natalia Duque, Esther Artime and Silvia Díaz report ownerships of Eli Lilly shares. Jesús Reviriego reports being an Eli Lilly and Company employee until January 17 2020, and a minor shareholder in Eli Lilly and Company. Xavier Mundet reports personal fees from Boehringer Ingelheim Pharmaceuticals Inc., Eli Lilly Company and Merck Sharp & Dohme. Juan J. Gorgojo-Martinez reports personal fees from Astra-Zeneca, Abbott, AbbVie Inc, Boehringer Ingelheim Pharmaceuticals Inc., Esteve, Janssen Pharmaceuticals, Eli Lilly and Company, Merck Sharp & Dohme, Novo-Nordisk, Pfizer, Roche Pharma, Sanofi-Aventis and Mundipharma, outside the submitted work. Jeremie Lebrec reports personal fees from Eli Lilly, during the conduct of the study. Enrique Carretero-Anibarro and Antoni Sicras-Mainar have no conflicts of interest to declare.
Compliance with Ethics Guidelines
This study was approved by the ethics committee of the Hospital de Tarrasa in Barcelona (study reference number: 02-19-399-039).
Data Availability
The datasets (aggregated and anonymised), analysed for this specific study, are available (on reasonable request) at the following email address: ansicras@atryshealth.com
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Duque, N., Artime, E., Romera, I. et al. Real-World Use of Insulin Glargine U100 and U300 in Insulin-Naïve Patients with Type 2 Diabetes Mellitus: DosInGlar Study. Adv Ther 38, 3857–3871 (2021). https://doi.org/10.1007/s12325-021-01773-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01773-z