Abstract
Introduction
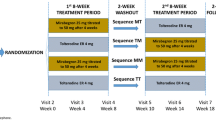
MATCH was a randomized, double-blind, placebo-controlled study enrolling Japanese and Korean men aged ≥ 40 years who still had overactive bladder (OAB) symptoms while receiving tamsulosin. After a 4-week single-blind screening period in which patients received placebo and tamsulosin, patients were randomized to mirabegron 50 mg + tamsulosin or placebo + tamsulosin for 12 weeks (n = 568). This post hoc analysis investigated the proportion of treatment responders for each treatment group and for subgroups stratified by age based on voiding diaries and patient-reported outcomes (PROs).
Methods
Responders were defined as those achieving normalization or clinically meaningful improvements in efficacy, or clinically important differences in PROs [≥ 10-point improvement in OAB questionnaire (OAB-q) symptom bother or total health-related quality of life (HRQoL) subscales at end of treatment (EoT; minimally important difference [MID]) or OAB symptom score (OABSS) total score decreased by ≥ 3 points at EoT [minimally clinically important change (MCIC)]].
Results
At EoT, micturition frequency normalization was achieved by 30.7% of tamsulosin + mirabegron patients and 18.6% of tamsulosin + placebo patients. Normalization of urgency and incontinence was 19.1% and 60.7% for tamsulosin + mirabegron and 18.2% and 60.0% for tamsulosin + placebo. Normalization of OAB symptoms based on OABSS was 17.1% for tamsulosin + mirabegron and 14.5% for tamsulosin + placebo. Higher proportions of patients in the mirabegron add-on group versus the placebo group reported clinically meaningful improvements in micturitions, urgency, and incontinence and in MCIC for OABSS and MID for the OAB-q subscales. Double- and triple-responder findings were as predicted by the results of single-responder analyses. These results were mirrored in the age groups using cut-offs of 65 and 75 years.
Conclusion
Mirabegron therapy added on to tamsulosin resulted in a higher frequency of responders in terms of normalization (e.g., micturition frequency normalization), clinically meaningful improvements in efficacy (e.g., ≥ 50% decrease in urgency), and minimally important changes in PROs (e.g., MCIC in OABSS).
Trial Registration
ClinicalTrials.gov identifier, NCT02656173.
Graphical Plain Language Summary


Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Previous results from the MATCH study showed that mirabegron therapy added on to tamsulosin was superior to placebo in improving objective endpoints, such as mean number of micturitions/24 h and mean volume voided/micturition, and also patient-reported outcomes (PROs), such as the overactive bladder symptom score (OABSS) and overactive bladder questionnaire (OAB-q) score. |
For a symptom-defined condition such as OAB, it is important to show that treatment effects are clinically meaningful to patients. |
Therefore, this analysis aimed to understand not only whether mirabegron treatment resulted in a change in OAB symptoms, but also whether this change was meaningful to patients for each symptom by investigating the proportion of treatment responders from the MATCH study and identifying responders by symptom resolution (normalization) or clinically important improvements. |
What was learned from the study? |
Mirabegron therapy added on to tamsulosin in men improved OAB symptoms with the most improvements seen in micturitions; these changes were meaningful and worthwhile to patients. |
Patients with OAB often have a constellation of symptoms, and the degree of distress due to individual symptoms varies. In addition, responsiveness can be different in individual patients. Considering that OAB patients have heterogeneous symptoms and concerns, it is important to understand treatment response in specific patient populations. |
Digital Features
This article is published with digital features, including a summary slide and graphical plain language summary, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13014158.
Introduction
Lower urinary tract symptoms (LUTS), including those of overactive bladder (OAB), occur commonly in older men. OAB is a highly prevalent syndrome, with an estimated 12.4% of the Japanese population ≥ 40 years of age experiencing symptoms [1]. Alpha-1 blockers are generally used as first-line pharmacotherapy for male LUTS, based on clinical guidelines for male LUTS and benign prostatic hyperplasia in Japan, which recommend that α1-blockers (or phosphodiesterase 5 inhibitors) be used regardless of prostate size [2]. However, since α1-blockers alone often fail to improve storage symptoms, including OAB, combination therapy of an α1-blocker plus an antimuscarinic has been recommended.
Mirabegron, a β3-adrenoreceptor agonist, is an alternative treatment option to antimuscarinics for OAB symptoms, with proven efficacy in both sexes [3, 4]. Two previous studies in Japan of mirabegron treatment added to tamsulosin have shown mirabegron add-on treatment to be effective and well tolerated [5, 6].
MATCH was a 12-week randomized, double-blind, placebo-controlled study at 53 sites in Japan and 5 sites in Korea, enrolling male patients aged ≥ 40 years who still had OAB symptoms while receiving tamsulosin for LUTS [7]. The primary objective was to evaluate the efficacy of mirabegron compared with placebo in men with OAB who were receiving treatment with the α1-blocker tamsulosin for LUTS. After a 4-week single-blind screening period in which patients received tamsulosin at a dose of 0.2 mg (in accordance with Japanese and Korean guidelines) + placebo, patients were randomized to tamsulosin + mirabegron 50 mg or tamsulosin + placebo for 12 weeks. The primary endpoint was change from baseline to end of treatment (EoT) in mean number of micturitions/24 h, based on a 3-day voiding diary. Secondary endpoints included change from baseline to EoT in mean number of urgency, urgency urinary incontinence, and incontinence episodes/24 h and in nocturia episodes, mean volume voided/micturition, and patient-reported outcomes (PROs), including the OAB symptom score (OABSS). Results, presented in the primary paper [7], showed that mirabegron add-on therapy was superior to placebo in improving the primary endpoint [adjusted mean difference (95% confidence interval [CI]) vs. placebo − 0.52 (− 0.82 to − 0.21)] and secondary endpoints. These included mean volume voided/micturition [12.08 (6.33–17.84)], OABSS [− 0.65 (− 1.04 to − 0.26)], International Prostate Symptom Score (IPSS) total [− 1.19 (− 1.94 to − 0.44)], IPSS storage [− 0.78 (− 1.13 to − 0.43)], IPSS quality of life scores [− 0.29 (− 0.51 to − 0.07)], OAB questionnaire (OAB-q) symptom bother [− 4.52 (− 6.91 to − 2.13)], and OAB-q total health-related quality of life [HRQoL; 2.79 (1.13–4.44)] at EoT (week 12). Differences compared with placebo in urgency, urgency urinary incontinence, and nocturia were not statistically significant [7].
For a symptom-defined condition such as OAB, PRO measures are important for evaluating disease severity and treatment efficacy [8]. In addition, it is important to show that treatment effects are clinically meaningful to patients. Responder analyses, based on identifying efficacy responders by symptom resolution (normalization) or by clinically important improvements, can provide support for results showing improvements in objective measures. This article reports results from a post hoc analysis that was conducted to investigate the proportion of responders from the MATCH study based on voiding diaries and PROs. The impact of age was also analyzed, using cut-offs of 65 years and 75 years.
Methods
In this post hoc analysis, responder analyses, including ten based on objective efficacy outcomes and two based on PROs related to HRQoL, were selected for inclusion. In line with the International Continence Society (ICS) guidelines for the presentation of data, symptom resolution/normalization rates were examined [9]. Normalization of micturitions was defined as fewer than eight micturition episodes/24 h at EoT, since eight or more micturitions/24 h are associated with a significant negative impact on symptom bother and HRQoL compared with fewer than eight micturitions/24 h [10]. For urgency, incontinence, urgency urinary incontinence, and nocturia, symptom normalization was defined as the complete absence of symptoms at EoT that had been present at baseline. Normalization of OABSS was defined as OABSS total score < 3 or OABSS Question 3 score < 2 at EoT.
Also examined were responders in terms of clinically meaningful improvements in efficacy: ≥ 50% decrease in urgency episodes/24 h at EoT, ≥ 50% decrease in incontinence episodes/24 h at EoT, ≥ 10% decrease in micturitions/24 h at EoT, and fewer than two urgency episodes/24 h at EoT.
Additional responder analyses included clinically important differences in PROs. Minimally important differences (MIDs) are defined as the smallest difference in score in the domain of interest that patients perceive as beneficial and that would mandate, in the absence of troublesome side effects and excessive cost, a change in patient management [11]. The MID for the OAB-q is 10 points [12]; therefore, a responder was defined by a ≥ 10-point decrease in OAB-q symptom bother or a ≥ 10-point increase in OAB-q HRQoL. The minimally clinically important change (MCIC) in OABSS was defined as OABSS total score decreased by ≥ 3 points at EoT [13].
Double and triple responders were also recorded. Double responders were patients who were simultaneously responders for micturitions, urgency, incontinence, urgency urinary incontinence, nocturia, or OABSS, AND OAB-q symptom bother OR OAB-q HRQoL. Triple responders were the proportion of patients who were simultaneously responders for micturitions, urgency, incontinence, urgency urinary incontinence, nocturia, or OABSS, AND OAB-q symptom bother AND OAB-q HRQoL. Responders were also recorded by age group (< 65 years vs. ≥ 65 years; < 75 years vs. ≥ 75 years). For each study site, approvals were obtained from the International Review Board/Ethics Committee before the start of this study. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All subjects provided informed consent to participate in the study.
Statistical Analysis
Efficacy assessments were based on the full analysis set (FAS), which included all patients who received at least one dose of double-blind study drug and had a baseline efficacy measurement and at least one postbaseline efficacy measurement. The number and percentage of patients who achieved responder criteria at EoT were summarized in each treatment group. Assessments by age group were also performed.
Results
Patients
A total of 779 patients provided written informed consent and entered the screening period. Of these, 730 patients received tamsulosin + placebo, as described previously [7]. In the treatment period, 568 patients were randomized to either tamsulosin + placebo or tamsulosin + mirabegron. Two patients did not take the study drug during the treatment period. In addition, one patient had no evaluable postbaseline efficacy data. In total, 544 patients completed the study (272 in the placebo group, 272 in the mirabegron 50 mg group). Of 565 patients in the FAS, 56.6% were ≥ 65 years (Table 1), and 14.7% were ≥ 75 years. In total, 23.4% of mirabegron patients reported ≥ 1 treatment-emergent adverse event (TEAE) compared to 22.5% of patients in the placebo group, and 3.9% and 6.3%, respectively, reported drug-related TEAEs.
Post Hoc Analyses
In terms of normalization, a higher frequency of responders was observed for the tamsulosin + mirabegron group than for the tamsulosin + placebo group (Fig. 1). The proportion of patients achieving micturition frequency normalization at EoT was 30.7% for tamsulosin + mirabegron compared with 18.6% for tamsulosin + placebo. Normalization of urgency was 19.1% for tamsulosin + mirabegron and 18.2% for tamsulosin + placebo; normalization of incontinence was 60.7% for tamsulosin + mirabegron and 60.0% for tamsulosin + placebo. Regarding urgency urinary incontinence, normalization was 64.0% for tamsulosin + mirabegron compared with 59.5% for tamsulosin + placebo. For nocturia, normalization was 10.6% for tamsulosin + mirabegron compared with 9.8% for tamsulosin + placebo. Normalization of OAB symptoms based on OABSS was 17.1% for the tamsulosin + mirabegron group and 14.5% for the tamsulosin + placebo group.
Similarly, higher proportions of patients in the mirabegron add-on group reported clinically meaningful improvements than those in the placebo group. The proportion of patients reporting ≥ 50% decrease in urgency episodes and incontinence episodes was 62.6% and 76.4%, respectively, for tamsulosin + mirabegron and 52.9% and 75.0%, respectively, for tamsulosin + placebo (Fig. 2i). The proportion of patients with ≥ 10% decrease in micturitions/24 h at EoT and fewer than two urgency episodes at EoT was greater for tamsulosin + mirabegron (58.8% and 61.5%, respectively) than for tamsulosin + placebo (46.8% and 51.1%, respectively; Fig. 2ii). Percentages achieving MIDs for OAB-q symptom bother and HRQoL subscales were 65.2% and 39.1% for tamsulosin + mirabegron and 54.6% and 31.8% for tamsulosin + placebo (Fig. 3i and ii). Percentages achieving the MCIC for the OABSS (≥ 3-point decrease in OABSS score) were 51.6% for tamsulosin + mirabegron and 42.0% for tamsulosin + placebo (Fig. 3iii).
Proportion of patients achieving (i) MID in OAB-q symptom bother score, (ii) MID in OAB-q HRQoL score, and (iii) MCIC in OABSS at EoT. EoT end of treatment, HRQoL health-related quality of life, MCIC minimally clinically important change, MID minimally important difference, OAB-q overactive bladder questionnaire, OABSS overactive bladder symptom score
Double- and triple-responder findings were as predicted by the results of single-responder analyses, with numerical differences observed in the combinations that achieved numerical differences in single-responder criteria. There were numerical differences in favor of the tamsulosin + mirabegron group for micturitions (normalization or ≥ 10% decrease in micturitions) and OAB-q symptom bother score OR OAB-q HRQoL; for ≥ 50% decrease in urgency and OAB-q symptom bother score OR OAB-q HRQoL; and for OABSS (normalization/MCIC) and OAB-q symptom bother score OR OAB-q HRQoL; Table 2. There was little numerical difference between the tamsulosin + mirabegron and tamsulosin + placebo groups for urgency normalization and OAB-q symptom bother score OR OAB-q HRQoL, although the proportions were numerically higher for the tamsulosin + placebo group than for the tamsulosin + mirabegron group.
Analysis by Age Group
In general, a higher frequency of responders was observed in both age groups for the tamsulosin + mirabegron group than for the tamsulosin + placebo group. Patients aged ≥ 65 years generally had similar responder rates to patients aged < 65 years. However, the proportion of tamsulosin + mirabegron patients achieving the OAB-q HRQoL MID was lower for those aged ≥ 65 years (33.3%) than for those aged < 65 years (47.0%; Table 3). Moreover, in the analyses by age < 75 years versus ≥ 75 years, although more responders were observed in both age groups for the tamsulosin + mirabegron group than for the tamsulosin + placebo group, responder rates themselves were more divergent among age groups for some endpoints. Double- and triple-responder analyses for each age category generally showed greater proportions for the tamsulosin + mirabegron group than for the tamsulosin + placebo group (Table 4). However, for urgency normalization AND OAB-q symptom bother score, the tamsulosin + placebo group showed numerically higher values than the tamsulosin + mirabegron group in all age groups except for < 65 years.
Discussion
In this post hoc analysis of male patients, results were consistent with those of the primary analysis, with a higher frequency of responders observed for most endpoints for the tamsulosin + mirabegron group than for the tamsulosin + placebo group. There were numerical differences in favor of the mirabegron add-on group for micturition endpoints (micturition normalization and ≥ 10% decrease in micturitions/24 h at EoT). In addition, numerical differences in favor of the mirabegron add-on group were observed for two of the urgency endpoints (≥ 50% decrease in urgency episodes/24 h at EoT and fewer than two urgency episodes/24 h at EoT). The MCIC for OABSS was also achieved, and this is likely the most relevant result for clinical practice [14] because the OABSS is simple and dependable, can provide a quick overall assessment of OAB symptoms, and can indicate symptom severity and bother [15, 16]. Both urgency and incontinence were more affected by increased age. For example, normalization of incontinence episodes in the placebo group ≥ 75 years was 69.2% (versus 66.7% in the mirabegron group).
Differences between the tamsulosin + mirabegron and the tamsulosin + placebo groups were not so pronounced for normalization of urgency, incontinence, urgency urinary incontinence, and nocturia, and for ≥ 50% decrease in incontinence episodes/24 h at EoT. However, in all instances, the tamsulosin + mirabegron group had a higher frequency of responders than the tamsulosin + placebo group. Regarding double responders, there were numerical differences in favor of tamsulosin + mirabegron versus tamsulosin + placebo for micturitions and 50% decrease in urgency, or OABSS and OAB-q (symptom bother or HRQoL). However, there was little numerical difference between the tamsulosin + mirabegron and tamsulosin + placebo groups for urgency normalization and OAB-q (symptom bother or HRQoL).
Another study has similarly found improved responder rates for mirabegron-treated patients, including improvements in micturitions and incontinence. In a post hoc analysis of pooled PRO data in phase 3 studies of mirabegron monotherapy (n = 1324) compared with placebo (n = 1328), mirabegron demonstrated greater improvements from baseline to EoT for responder analyses, whether for individual objective and subjective outcomes or for double and triple responders [17].
Double responders were patients who were simultaneously responders for either incontinence or micturitions as well as PROs [OAB-q symptom bother scale, OAB-q total HRQoL, or patient perception of bladder condition (PPBC) scale]. Triple responders were patients who were simultaneously responders for either incontinence or micturitions as well as PPBC and the OAB-q symptom bother scale, or PPBC and OAB-q total HRQoL. Improvements over placebo were statistically significant for all double- and triple-responder analyses and for all single-responder analyses except for PPBC.
A recent study also reported improved symptoms for mirabegron in combination with a phosphodiesterase 5 inhibitor, tadalafil 5 mg (n = 89), compared with tadalafil monotherapy (n = 87) in male patients aged ≥ 50 years with residual OAB symptoms [18]. The primary endpoint, change from baseline in total OABSS, was statistically significantly decreased by 1.78 (95% CI 1.05–2.50) points in the combination group compared with the monotherapy group (P < 0.001). Changes in OABSS nighttime voiding score, urgency score, urgency urinary incontinence score, IPSS storage subscores, and micturition chart variables (number of voids, nighttime voids, and urgency episodes/24 h) were also significantly reduced in the combination group (all P < 0.001).
It is possible that antimuscarinics have a more pronounced effect than mirabegron on incontinence and also that antimuscarinics have a more pronounced effect on urgency than on micturitions. The latter certainly seemed to be the case in the ASSIST and SOFIA studies, although it should be noted that these studies had a different primary endpoint than that of MATCH, so cannot be directly compared.
ASSIST was a randomized, double-blind study, in which 638 men aged ≥ 50 years with residual OAB symptoms despite tamsulosin monotherapy were randomized to 12 weeks of add-on therapy with solifenacin (2.5 mg or 5 mg) or placebo [19]. The primary endpoint, mean change in urgency episodes, was reduced by 2.2 episodes in the tamsulosin + solifenacin 2.5 mg group and 2.4 episodes in the tamsulosin + solifenacin 5 mg group, with differences achieving statistical significance compared with tamsulosin + placebo. The number of micturitions/24 h was reduced by 1.27 episodes in the tamsulosin + solifenacin 2.5 mg group and 1.06 episodes in the tamsulosin + solifenacin 5 mg group, once again with statistically significant differences compared with tamsulosin + placebo. The number of nocturia episodes and urgency urinary incontinence episodes were numerically reduced in both tamsulosin + solifenacin groups versus tamsulosin + placebo; however, these differences did not reach statistical significance. Although responder rates were not reported, a statistically significant reduction of OABSS urgency score and number of micturitions/24 h was shown in both solifenacin add-on groups compared with the placebo group, whereas a significant reduction of urgency episodes from bladder diary entries was demonstrated in only the tamsulosin + solifenacin 5 mg group.
Results from a 12-week randomized, placebo-controlled trial (SOFIA) in 794 patients showed that fesoterodine was associated with statistically significantly and clinically greater improvements in urgency episodes (primary endpoint), micturition frequency, incontinence pad use, nocturia, and PROs than placebo in adults ≥ 65 years with OAB, but not in urgency urinary incontinence episodes [20]. Mean number of urgency episodes/24 h decreased from 8.5 at baseline to 4.6 at week 12 in the fesoterodine group and from 8.8 to 6.3 in the placebo group. Mean number of micturitions/24 h decreased from 11.9 to 9.8 in the fesoterodine group and 12.1 to 10.9 in the placebo group. Mean number of incontinence pads used/24 h decreased from 2.8 to 1.8 in the fesoterodine group and 3.3 to 2.7 in the placebo group. Mean number of nocturia episodes decreased from 2.8 to 2.2 in the fesoterodine group and 2.9 to 2.6 in the placebo group. Responder rates on the treatment benefit scale, PPBC, urgency perception scale, and OAB-q satisfaction were also statistically significantly higher with fesoterodine.
However, in a noninferiority study comparing efficacy and safety of mirabegron and solifenacin in 1887 OAB patients dissatisfied with previous antimuscarinic treatment due to lack of efficacy (the BEYOND study), both mirabegron and solifenacin improved responder rates [21]. Micturition normalization at EoT was achieved in 43.6% and 47.2% of mirabegron and solifenacin patients, respectively, with a corresponding odds ratio (OR) versus mirabegron (95% CI) of 1.14 (0.93–1.39); 85.1% and 88.1% of patients, respectively, achieved a ≥ 50% decrease in incontinence episodes/24 h at EoT with a corresponding OR (95% CI) versus mirabegron of 1.25 (0.82–1.90), and 67.3% and 68.5% of patients, respectively, achieved zero incontinence episodes at EoT, OR (95% CI) versus mirabegron 1.02 (0.73–1.42). None of the treatment differences were statistically significant.
The proportion of responders in the mirabegron add-on group in the current MATCH study is similar to the single-responder rates from the BESIDE study, in which mirabegron was used as add-on treatment to the antimuscarinic solifenacin in 2174 patients (male and female) with OAB wet and an inadequate response to solifenacin monotherapy [22]. Compared with solifenacin 5 mg and 10 mg monotherapy, patients receiving mirabegron add-on treatment were 47% and 28% more likely to achieve zero incontinence and 51% and 25% more likely to achieve ≥ 50% decrease in incontinence episodes/24 h. They were also 29% and 12% more likely, respectively, to achieve normalization of micturition frequency. There was statistical significance in favor of the combination compared with solifenacin 5 and 10 mg in the proportion of responders with a ≥ 10-point improvement in OAB-q symptom bother score or OAB-q total HRQoL and a major (≥ 2 point) improvement in PPBC [22]. At EoT, statistically significant improvements were demonstrated for all five exploratory variables (three double- and two triple-responder analyses) in favor of the combination group compared with solifenacin 5 mg.
SYNERGY was a study comparing solifenacin 5 mg + mirabegron 25 mg and solifenacin 5 mg + mirabegron 50 mg with monotherapy and placebo in both treatment-naive and previously treated patients (male and female) with OAB wet (n = 3398). In this study, odds ratios in favor of both combined therapies were shown for the proportion of patients achieving incontinence and micturition frequency normalization compared with monotherapies [23]. In addition, OAB-q symptom bother score responder rates (≥ 10-point improvement from baseline to EoT) were statistically significantly higher than for mirabegron monotherapy for both combination groups, and the combination 5 + 50 mg was significantly better than solifenacin monotherapy [24]. The highest proportion of double responders (50% decrease in incontinence and ≥ 10-point improvement in OAB-q symptom bother) was observed in the combination groups.
The PREFER study, a double-blind, randomized, crossover study in which 358 patients of both sexes received mirabegron and tolterodine, included responder rates for OAB-q subscales and OAB Satisfaction (OAB-S) Medication Tolerability score ≥ 90 [25]. The percentage of responders at EoT for mirabegron and tolterodine extended release was 71.7% versus 65.5% for OAB-q symptom bother and 59.3% versus 54.2% for OAB-q total HRQoL. For the OAB-S medication tolerability score, the percentage of responders (score ≥ 90) at EoT was 52.5% for mirabegron and 48.5% for tolterodine extended release.
In a postmarketing study of mirabegron in 9795 patients aged ≥ 75 years with OAB, mean total OABSS decreased significantly from baseline and exceeded the MCIC in 61.0% and 65.9% of patients aged ≥ 75 and < 75 years, respectively [26]. However, it should be noted that the BESIDE, SYNERGY, and PREFER studies and the postmarketing study all enrolled more female patients than male patients with OAB.
Responder analyses provide a tool to translate changes in subjective or objective measures into clinically meaningful binary outcomes—responders or nonresponders. In this study, responder analyses confirmed that the patients with OAB who achieved significant reductions in symptoms also experienced significant benefits in HRQoL. Higher proportions of patients in the mirabegron add-on group versus the placebo group reported clinically meaningful improvements in the MCIC for OABSS and the MID for the OAB-q subscales, illustrating that add-on therapy has the potential to lead to higher treatment success.
Limitations, due to the post hoc nature of this analysis, are that differences between groups were not analyzed statistically and the PPBC not measured. Further limitations are the omission of severe urgency (PPIUS grade 3 or 4) as an inclusion criterion and the unbalanced ratio of patients by country. In addition, future studies might benefit from including a tamsulosin plus antimuscarinic comparative arm.
Conclusion
Mirabegron therapy added on to tamsulosin in men resulted in a higher frequency of responders compared with the tamsulosin + placebo group in terms of normalization (e.g., micturition frequency normalization), clinically meaningful improvements in efficacy (e.g., ≥ 50% decrease in urgency), and minimally important changes in PROs (e.g., MCIC in OABSS). These results were mirrored in the age groups using cut-offs of 65 years and 75 years.
References
Homma Y, Yamaguchi O, Hayashi K, Neurogenic Bladder Society Committee. An epidemiological survey of overactive bladder symptoms in Japan. BJU Int. 2005;96(9):1314–8. https://doi.org/10.1111/j.1464-410X.2005.05835.x.
Homma Y, Gotoh M, Kawauchi A, et al. Clinical guidelines for male lower urinary tract symptoms and benign prostatic hyperplasia. Int J Urol. 2017;24(10):716–29. https://doi.org/10.1111/iju.13401.
Chapple CR, Cardozo L, Nitti VW, Siddiqui E, Michel MC. Mirabegron in overactive bladder: a review of efficacy, safety, and tolerability. Neurourol Urodyn. 2014;33(1):17–30. https://doi.org/10.1002/nau.22505.
Tubaro A, Batista JE, Nitti VW, et al. Efficacy and safety of daily mirabegron 50 mg in male patients with overactive bladder: a critical analysis of five phase III studies. Ther Adv Urol. 2017;9(6):137–54. https://doi.org/10.1177/1756287217702797.
Wada N, Iuchi H, Kita M, Hashizume K, Matsumoto S, Kakizaki H. Urodynamic efficacy and safety of mirabegron add-on treatment with tamsulosin for Japanese male patients with overactive bladder. Low Urin Tract Symptoms. 2016;8(3):171–6. https://doi.org/10.1111/luts.12091.
Ichihara K, Masumori N, Fukuta F, Tsukamoto T, Iwasawa A, Tanaka Y. A randomized controlled study of the efficacy of tamsulosin monotherapy and its combination with mirabegron for overactive bladder induced by benign prostatic obstruction. J Urol. 2015;193(3):921–6. https://doi.org/10.1016/j.juro.2014.09.091.
Kakizaki H, Lee KS, Yamamoto O, et al. Mirabegron add-on therapy to tamsulosin for the treatment of overactive bladder in men with lower urinary tract symptoms: a randomized, placebo-controlled study (MATCH). Eur Urol Focus. 2019. https://doi.org/10.1016/j.euf.2019.10.019.
Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87(9):760–6. https://doi.org/10.1046/j.1464-410x.2001.02228.x.
Mattiasson A, Djurhuus JC, Fonda D, Lose G, Nordling J, Stohrer M. Standardization of outcome studies in patients with lower urinary tract dysfunction: a report on general principles from the Standardisation Committee of the International Continence Society. Neurourol Urodyn. 1998;17(3):249–53. https://doi.org/10.1002/(sici)1520-6777(1998)17:3<249:aid-nau9>3.0.co;2-d.
Coyne KS, Payne C, Bhattacharyya SK, et al. The impact of urinary urgency and frequency on health-related quality of life in overactive bladder: results from a national community survey. Value Health. 2004;7(4):455–63. https://doi.org/10.1111/j.1524-4733.2004.74008.x.
Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–15. https://doi.org/10.1016/0197-2456(89)90005-6.
Coyne KS, Matza LS, Thompson CL, Kopp ZS, Khullar V. Determining the importance of change in the overactive bladder questionnaire. J Urol. 2006;176(2):627–32. https://doi.org/10.1016/j.juro.2006.03.088(discussion 32).
Gotoh M, Homma Y, Yokoyama O, Nishizawa O. Responsiveness and minimal clinically important change in overactive bladder symptom score. Urology. 2011;78(4):768–73. https://doi.org/10.1016/j.urology.2011.06.020.
Leidy NK, Revicki DA, Geneste B. Recommendations for evaluating the validity of quality of life claims for labeling and promotion. Value Health. 1999;2(2):113–27. https://doi.org/10.1046/j.1524-4733.1999.02210.x.
Homma Y, Kakizaki H, Yamaguchi O, et al. Assessment of overactive bladder symptoms: comparison of 3-day bladder diary and the overactive bladder symptoms score. Urology. 2011;77(1):60–4. https://doi.org/10.1016/j.urology.2010.06.044.
Homma Y, Gotoh M. Symptom severity and patient perceptions in overactive bladder: how are they related? BJU Int. 2009;104(7):968–72. https://doi.org/10.1111/j.1464-410X.2009.08498.x.
Castro-Diaz D, Chapple CR, Hakimi Z, et al. The effect of mirabegron on patient-related outcomes in patients with overactive bladder: the results of post hoc correlation and responder analyses using pooled data from three randomized phase III trials. Qual Life Res. 2015;24(7):1719–27. https://doi.org/10.1007/s11136-014-0904-4.
Yamanishi T, Kaga K, Sakata K, et al. A randomized controlled study of the efficacy of tadalafil monotherapy versus combination of tadalafil and mirabegron for the treatment of persistent overactive bladder symptoms in men presenting with lower urinary tract symptoms (CONTACT Study). Neurourol Urodyn. 2020;39(2):804–12. https://doi.org/10.1002/nau.24285.
Yamaguchi O, Kakizaki H, Homma Y, et al. Solifenacin as add-on therapy for overactive bladder symptoms in men treated for lower urinary tract symptoms—ASSIST, randomized controlled study. Urology. 2011;78(1):126–33. https://doi.org/10.1016/j.urology.2011.02.055.
Wagg A, Khullar V, Marschall-Kehrel D, et al. Flexible-dose fesoterodine in elderly adults with overactive bladder: results of the randomized, double-blind, placebo-controlled study of fesoterodine in an aging population trial. J Am Geriatr Soc. 2013;61(2):185–93. https://doi.org/10.1111/jgs.12088.
Batista JE, Kolbl H, Herschorn S, et al. The efficacy and safety of mirabegron compared with solifenacin in overactive bladder patients dissatisfied with previous antimuscarinic treatment due to lack of efficacy: results of a noninferiority, randomized, phase IIIb trial. Ther Adv Urol. 2015;7(4):167–79. https://doi.org/10.1177/1756287215589250.
MacDiarmid S, Al-Shukri S, Barkin J, et al. Mirabegron as add-on treatment to solifenacin in patients with incontinent overactive bladder and an inadequate response to solifenacin monotherapy: responder analyses and patient-reported outcomes from the BESIDE study [corrected]. J Urol. 2016;196(3):809–18. https://doi.org/10.1016/j.juro.2016.03.174.
Herschorn S, Chapple CR, Abrams P, et al. Efficacy and safety of combinations of mirabegron and solifenacin compared with monotherapy and placebo in patients with overactive bladder (SYNERGY study). BJU Int. 2017;120(4):562–75. https://doi.org/10.1111/bju.13882.
Robinson D, Kelleher C, Staskin D, et al. Patient-reported outcomes from SYNERGY, a randomized, double-blind, multicenter study evaluating combinations of mirabegron and solifenacin compared with monotherapy and placebo in OAB patients. Neurourol Urodyn. 2018;37(1):394–406. https://doi.org/10.1002/nau.23315.
Herschorn S, Staskin D, Tu LM, et al. Patient-reported outcomes in patients with overactive bladder treated with mirabegron and tolterodine in a prospective, double-blind, randomized, two-period crossover, multicenter study (PREFER). Health Qual Life Outcomes. 2018;16(1):69. https://doi.org/10.1186/s12955-018-0892-0.
Yoshida M, Nozawa Y, Kato D, Tabuchi H, Kuroishi K. Safety and effectiveness of mirabegron in patients with overactive bladder aged >/=75 years: analysis of a Japanese post-marketing study. Low Urin Tract Symptoms. 2019;11(1):30–8. https://doi.org/10.1111/luts.12190.
Acknowledgements
The authors thank the MATCH study investigators and all patients and their parents/legal representatives who took part in the study.
Funding
The MATCH study and this analysis (study design and conduct, data collection, management, analysis and interpretation of the data, review and approval of the manuscript, and the Rapid Service and Open Access Fees) were funded by Astellas Pharma Inc.
Medical Writing Assistance
Medical writing support was provided by Sue Cooper of Elevate Scientific Solutions and funded by Astellas Pharma Global Development.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Hidehiro Kakizaki is a consultant, lecturer, and advisory board member for Astellas Pharma Inc.; consultant and advisory board member for Kyorin; speaker for Kissei, Nippon Shinyaku, and Pfizer; consultant for Taiho. Kyu-Sung Lee is a consultant for Astellas Pharma Inc. Daisuke Katou, Osamu Yamamoto, and Satoshi Uno are employees of Astellas Pharma Inc., Japan. Budiwan Sumarsono is an employee of Astellas Pharma, Singapore. Osamu Yamaguchi is a consultant, lecturer, and advisory board member for Astellas Pharma Inc.; lecturer for Kyorin and Pfizer; consultant for Taiho; consultant and lecturer for Hisamitsu and Asahi Kasei.
Compliance With Ethics Guidelines
For each study site, approvals were obtained from the International Review Board/Ethics Committee before the start of this study. This study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. All subjects provided informed consent to participate in the study.
Data Availability
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials at https://www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kakizaki, H., Lee, KS., Katou, D. et al. Mirabegron Add-On Therapy to Tamsulosin in Men with Overactive Bladder: Post Hoc Analyses of Efficacy from the MATCH Study. Adv Ther 38, 739–757 (2021). https://doi.org/10.1007/s12325-020-01517-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01517-5