Abstract
Introduction
Since sipuleucel-T approval in 2010, the treatment landscape for metastatic castration-resistant prostate cancer (mCRPC) now includes the androgen-receptor signaling pathway inhibitors (ASPIs) abiraterone acetate or enzalutamide. In 2013 and 2014, these oral agents were approved for use in men with metastatic prostate cancer who had minimal to no symptoms. We compared overall survival (OS) in men who received their first mCRPC treatment using the Medicare Fee-for-Service 100% administrative claims research dataset with patient-level linkage to the National Death Index.
Methods
This retrospective cohort analysis (January 2013 to December 2017) included men who were chemo-naïve at treatment start in 2014 and who had continuous Medicare Parts A, B, and D eligibility during the 3-year observation period. We compared: first-line sipuleucel-T vs. first-line ASPIs and any-line sipuleucel-T vs. any-line ASPIs (without sipuleucel-T). We used a multivariable regression model to help control for potentially confounding factors while assessing survival outcomes.
Results
The model included 6044 eligible men (average age 75–78 years) with similar disease severity; > 80% were white. Median OS, presented as sipuleucel-T vs. ASPI, was 35.2 vs. 20.7 months (n, 906 vs. 5092; any-line cohort) and 34.9 vs. 21.0 months (n, 647 vs. 4810; first-line cohort). Model outcomes indicated sipuleucel-T was associated with significantly prolonged OS compared with ASPIs: adjusted hazard ratio, 0.59 (95% CI 0.527–0.651) and 0.56 (0.494–0.627) for the any-line and first-line cohorts, respectively.
Conclusion
This analysis suggests use of sipuleucel-T at any time was associated with improved OS compared with ASPI use alone. Of note, these analyses are intended as descriptive rather than definitive as this dataset contains limited data on key clinical factors. While selection bias is a risk in secondary claims data, this research provides important insight into real-world treatment outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Survival outcomes of treatment of advanced prostate cancer with androgen-receptor signaling pathway inhibitors (ASPIs), abiraterone acetate and enzalutamide, or sipuleucel-T have not been compared in a prospective clinical trial. |
We addressed this data gap by generating multivariable models to analyze data from the large longitudinal Medicare 100% dataset linked to the National Death Index as it offers large numbers of patients. |
We hypothesized that patients receiving sipuleucel-T would have improved survival compared to non-sipuleucel-T users, potentially related to its distinct mechanism of action compared to other mCRPC directed therapies. |
What was learned from the study? |
Model outcomes indicated sipuleucel-T, regardless of line of use, was associated with significantly prolonged OS compared with ASPIs: adjusted hazard ratio, 0.59 (95% CI 0.527–0.651). |
Even given the potential limitations associated with claims analyses, such as selection bias and confounding by indication, this research provides important insights into real-world treatment outcomes and is complementary with other recently published real-world evidence analyses from other data sources. |
Digital Features
This article is published with digital features to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12961697.
Introduction
Castration-resistant prostate cancer (CRPC) is an advanced, aggressive form of prostate cancer characterized by disease progression despite castration by surgery or androgen deprivation therapy [1, 2]. Once metastatic CRPC (mCRPC) develops, the disease is inevitably fatal, although several drugs have been shown to prolong survival in men with advanced prostate cancer.
Sipuleucel-T is an autologous antigen-presenting cell vaccine, which is manufactured through co-culturing leukapheresed immune cells with a fusion protein consisting of prostatic acid phosphatase and granulocyte–macrophage colony-stimulating factor [3, 4]. Upon infusion, activated antigen-presenting cells contained in the sipuleucel-T product are thought to stimulate effector T-cells, which in turn result in an anti-tumor effect [4]. Since sipuleucel-T was approved for use in the US in 2010, the treatment landscape for patients with mCRPC has grown to include two androgen-receptor signaling pathway inhibitors (ASPIs), abiraterone acetate and enzalutamide, both initially approved in the same patient population as sipuleucel-T. Abiraterone and enzalutamide have become the most commonly used agents in treating mCRPC in the US [5]. Current NCCN guidelines for mCRPC treatment include both ASPIs and sipuleucel-T as first-line recommendations [6].
Research examining the survival outcomes of the second-generation ASPIs and sipuleucel-T in a large cohort of treated mCRPC patients has not been performed [7]. While a clinical trial can provide useful information, limitations include the understanding of real-world effectiveness of treatment in a heterogeneous population [8,9,10]. Real-world evidence research provides data that are complementary to clinical trials: effectiveness vs. efficacy and real-world risk:benefit assessments, to start [8,9,10]. Two commonly used sources of real-world evidence are health records and claims data.
In the US, healthcare for a life-threatening disease such as cancer, diagnosis, and treatment will typically occur across multiple medical professionals, pharmacies, and treatment centers leading to discontinuous medical records [10]. Electronic health records can offer a breadth of clinical data yet still with a certain level of missing data and percentage of patients being lost to follow-up. One example is a study published by George et al. [10]. This study described survival outcomes and treatment patterns using electronic health records from oncology clinics for men with mCRPC covered by a variety of payers and leaving outcomes from urology clinics [10].
One opportunity for longitudinal data in the US lay with claims data. One key claims dataset is the Medicare Fee-for-Service (FFS) 100% research identifiable longitudinal dataset, hereafter referred to as “Medicare 100%.” Medicare 100% includes longitudinal claims data from over 40 million patients, typically 65 years or older, and other patients who receive Medicare and possibly Medicaid through the Centers for Medicare and Medicaid Services (CMS). The data provide information about medical interventions for which claims were made. Furthermore, this research dataset is linked to the National Death Index to provide information on survival outcomes. Thus, the Medicare 100% dataset provides longitudinal claims data that can be linked to survival outcomes from a large contemporary patient population. A subset of patients included in this dataset are those who may have supplemental insurance through the Medicare Advantage plans. Given claims records may be subject to confounding by indication by treatment as a patient’s clinical condition at treatment start will influence survival outcome, several studies that use Medicare data have provided relevant clinical insights into prostate cancer treatment [11,12,13,14].
The objective of this study was to compare the effectiveness of sipuleucel-T and ASPIs on overall survival in men treated for advanced prostate cancer as captured in the Medicare 100% dataset. We chose to compare with ASPIs as they were not available when sipuleucel-T was approved, and they have become the most commonly used agents for treating mCRPC in the US. Given the risk of confounding by indication, we chose to study the subset of patients with advanced prostate cancer who were chemotherapy naïve at the start of each type of treatment and to use multivariate modeling to control for those factors that were assessable in this dataset. We used the Medicare dataset because it offers the type of longitudinal data needed. We hypothesized that patients receiving sipuleucel-T would have improved survival compared to non-sipuleucel-T users, potentially related to its distinct mechanism of action compared to other mCRPC directed therapies.
Methods
Data Source
This analysis used the Medicare 100% research dataset, including linked Parts A, B, and D claims and enrollment for all Medicare beneficiaries in the USA. This dataset includes longitudinal, anonymized demographic information and claims data (dates of service, diagnosis codes [International Classification of Diseases Clinical Modification 9th and 10th versions (ICD-9-CM and ICD-10-CM, respectively)] and procedure codes [current procedural terminology codes]) from hospitals and other institutional and non-institutional providers. Patient records were linked to the National Death Index to obtain dates of death, allowing for survival analysis. Dendreon Pharmaceuticals LLC engaged Milliman Inc. (New York, USA) to perform analytics on this dataset.
This retrospective study used the secondary database, the Medicare 100% research dataset, which is based on anonymized patient claims data. Dendreon and Milliman had permission to access and use these data. This research is exempt from institutional review board approval.
Study Population
The study population (the Overall Analysis Set) comprised a subset of the Medicare 100% beneficiary population, identified through the application of a set of prespecified criteria (see Fig. 1 for a detailed flow chart). During the entire observation period (2013–2017), men in the Overall Analysis Set had to be eligible for Medicare Parts A, B, and D without enrollment in a Medicare Advantage plan, the latter to ensure consistency in both coverage and follow-up. Identification of men with mCRPC required a qualifying prostate cancer diagnosis in 2014 (ICD-9-CM 185) and an initial claim for an approved mCRPC treatment in 2014, which predated approvals of mCRPC agents for hormone-sensitive prostate cancer. Identification of treatment agents was based on Healthcare Common Procedure Coding System (HCPCS) codes and national drug codes (NDCs). We further qualified our analysis set to include only mCRPC-treatment-naïve treated men, as confirmed by having no previous FDA-approved mCRPC treatment in the 12 months before the initial 2014 claim, with the exception of androgen-deprivation therapy (i.e., index date; see Supplemental Fig. 1 for a description). To minimize censoring, all patients were required either to have continuous coverage for 36 months or to have died.
Identification of eligible patients for the overall analysis set. This figure illustrates the impact of sequentially applying the specified eligibility criteria to the Medicare 100% Fee-for-Service beneficiary population to identify the final population used in the model (Overall Analysis Set). This population included men who were eligible for Medicare Parts A, B, and D without enrollment in a Medicare Advantage plan; who had a qualifying prostate cancer diagnosis in 2014 (ICD-9-CM 185); who had an initial claim for an approved mCRPC treatment in 2014 with no previous FDA-approved mCRPC treatment in the 12 months before the initial 2014 claim, with the exception of androgen-deprivation therapy (i.e., index date); and who had either available claims data for 36 months or had died during this time period
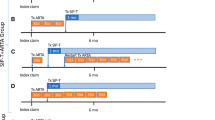
For this research, there were two analysis cohorts: first-line use or any-line use (Fig. 2). First, there was the first-line cohort: men who received first-line sipuleucel-T versus those who received first-line ASPIs (first-line sipuleucel-T versus first-line ASPIs). These patients could have received any other approved mCRPC agent in subsequent lines. Second, there was the any-line cohort that comprised two distinct groups: men who received sipuleucel-T at any time during the observation period and who could have received any other agent during the observation period versus those men who received ASPIs in any line and who could have received any other agent during the observation period except for sipuleucel-T (any-line sipuleucel-T versus any-line ASPIs).
Description of analysis sets. This figure describes the composition of the final analysis sets used in the model. Using the Overall Analysis Set (A), we identified those men who had ever received sipuleucel-T (B) or androgen-receptor signaling pathway inhibitors (ASPIs) (C) during the observation period from 2014 to 2017. We created the first-line cohort, comparing those men who received sipuleucel-T (D) with those who received ASPIs (E). In the first-line cohort, patients could have received any agent in subsequent lines. Next, we identified those who received sipuleucel-T (G) and those who received ASPIs (F) at any time. However, since these two groups (F, G) included some of the same men, we excluded those men who received sipuleucel-T (I) from the larger group of those who received ASPIs (F) to prevent overlapping. The any-line cohort thus compares men who, during the observation period, received sipuleucel-T at any time (G) with those who received ASPIs, but never received sipuleucel-T (H). The resultant first-line and any-line cohorts were used in the models to determine the impact of each type of treatment
As an exploratory outcome on sequencing with sipuleucel-T and ASPI, we compared the overall survival of patients using a first- or second-line sequence of sipuleucel-T with the ASPIs (without consideration of third-line treatments).
Analytical Methods
Following common statistical modeling principles, we developed a multivariable model using Cox (proportional hazard) methods to analyze survival outcomes while controlling for known (potential) confounding variables and minimizing selection bias. For each model, the covariate and model fit statistics and hazard ratios (HRs) were calculated and assessed. Initially, univariate analyses were performed to explore overall survival (OS) in this population [15].
Next, we developed a multivariable model using a stepwise procedure. First, multiple models were developed using two-thirds of the population of the Overall Analysis Set (described in Supplemental Table 1). To identify the best fit, we varied type of selection (forward, backward, and stepwise), significance level (0.05 and 0.01), observation period (36 months), and cohort (first line vs. any line) (described in Supplemental Table 2) and evaluated a prespecified list of covariates (Supplemental Table 3). These covariates include a selection of known clinical confounders [16] that can be identified using claims data as well as their variations. For example, clinical confounders included claim codes indicating presence of metastases and skeletal-related events (SREs), explored either as present or not, or by specific site. Additionally, socioeconomic factors such as location (rural or urban), household income, Centers for Disease Control region, type of coverage (Medicare alone or Medicare plus Medicaid), and Hierarchical Condition Category (HCC) community score (a way to incorporate risks associated with comorbidities) were assessed. The various initial models demonstrated very good concordance with each other, with high overlap between significant covariates.
The model selected to move forward was the stepwise model, with a significance level of 0.05, for the any-line cohort. The final selection of covariates included treatments received (sipuleucel-T vs. ASPI), number of lines of treatment, age, race, type of coverage, Charlson comorbidity index score [17], number of sites with metastatic disease, prior SREs, chronic opioid use (as a surrogate for disease severity), numbers of lines of therapy, and corticosteroid use [18,19,20]. For a detailed description of these covariates, please see Supplemental Table 4. Medication use (i.e., mCRPC treatments, oral corticosteroids, and opiates) was identified through NDC codes. Note, corticosteroid use excluded concomitant use with abiraterone as per label. Presence of metastases was assumed if ICD-9-CM diagnosis codes indicating such were present during the year before the index date (the date of the first claim for mCRPC treatment). SREs were defined based on claims for bone fracture, bone surgery, or spinal cord compression in the 90-day window before or after the index date and/or radiation therapy within a 60-day window. Charlson comorbidity index scores were calculated based on established methods [17]. Patients with missing data in expected fields were excluded. We tested the model using the remaining one-third of patients from the Overall Analysis Set and the identified covariates. The characteristics for these men (Supplemental Table 5) were highly homogeneous with the initial population. Model success was based on a comparison of modeling the training and validation populations as measured by the concordance (C) statistic; the closer the C value is to 1 indicates better concordance. The initial and testing C-statistics were 0.7331 and 0.7618, respectively.
Last, we applied the model to the complete patient population (i.e., Overall Analysis Set), which is described in Table 1. The model proved robust and consistent. Direct adjusted survival functions were calculated and graphed for the models comparing the agents [21].
In lieu of having safety data, we report the frequency of emergency department visits, both overall visits and prostate cancer-related visits, that occurred within the first year and are reported according to first line therapy. Given the nature of claims data, clinical details are limited.
For the frequencies of the use the mCRPC agents by line of therapy, we identified the use of agents using HCPCS codes (for medical claims) and national drug codes (NDCs) (for Part D claims). Lines of therapy were determined as the earliest claim for each unique mCRPC agent without consideration of concurrent or layered utilization of products.
For both the exploratory analysis and the estimation of survival for the overall analysis set, we performed univariate Kaplan-Meier survival analyses comparing survival outcomes and calculating unadjusted HRs for either the sequence of sipuleucel-T as first-line followed by ASPIs or the sequence of ASPIs as first-line followed by sipuleucel-T compared to both ASPIs in a sequence or only one ASPI.
Statistical significance in this study was set at P ≤ 0.05. All reported P values are two-sided. Analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
We examined the Medicare 100% research dataset (2013 through 2017), using an index year of 2014 and a total population of 40,569,828 beneficiaries. A diagram detailing the identification of eligible patients is provided in Fig. 1. Of the 6,034,317 men having continuous Medicare Parts A, B, and D eligibility without enrollment in a Medicare Advantage plan during the observation period, 452,718 had a diagnosis of prostate cancer in 2014. Of the 14,351 who had ≥ 1 claim for mCRPC treatment in 2014, 6800 had no mCRPC treatment claim in the previous 12 months. In the end, we identified 6044 men with a prostate cancer diagnosis who received either sipuleucel-T or ASPIs (Overall Analysis Set).
The patients identified in the Overall Analysis set were then assigned to specific cohorts as described in Fig. 2. The first study cohort included 647 men receiving first-line sipuleucel-T vs. 4810 receiving first-line ASPIs. Similarly, the second study cohort compared 906 men who received sipuleucel-T vs. 5092 men who received ASPIs but never received sipuleucel-T.
Patient Characteristics
The populations in the two cohorts were similar with the exception of minor differences between patients in the sipuleucel-T and ASPI arms in each cohort; these differences were in those factors included as covariates in the multivariable model (Table 1). Patients receiving ASPIs were slightly older on average (78 years) than those receiving sipuleucel-T (75 years). Most patients were white (88% and 83%, respectively). Most were only eligible for Medicare (93% and 86%, respectively), with slightly more men receiving ASPIs being covered by both Medicare and Medicaid, typically an indicator of the presence of comorbidities warranting dual coverage. Other variables reflecting prognosis, disease severity, and comorbidities were similar across groups (Table 1). As these factors were included in the covariates in the multivariable model, separate statistical testing was not done.
Treatments
Looking at the number of lines of therapy during the 36-month window for the any-line cohort, most men (75%) receiving ASPIs in any line without sipuleucel-T had one or two lines of therapy, whereas 75% of those receiving sipuleucel-T at any time received two to three lines of therapy (Table 1). Among men who received sipuleucel-T, most (71%) received it in the first line.
Figure 3 illustrates the frequencies of mCRPC treatments by line of therapy. In this population, the most frequently used agents in first line are abiraterone (56%), enzalutamide (24%), and sipuleucel-T (11%). Sixty-two percent of men (3744 of 6044) continued on to receive second-line therapy. In the second line, enzalutamide and abiraterone switch relative frequencies (48% versus 25% of these men) and then continue to decrease in frequency of use with each successive line. Sipuleucel-T use was observed in up to the fourth line.
Patients receiving treatment for metastatic castration-resistant prostate cancer by line of therapy. Frequency per agent is presented in descending order by line of therapy: a first line, b second line, c third line, d fourth line, and e fifth line. Agents identified as treatments for metastatic castration-resistant prostate cancer are included. The number of patients receiving treatment in each line is presented
Emergency Department Visits
Emergency department visits provide an insight into serious adverse events in claims databases, although clinical details may be limited. The average numbers of emergency department visits per 100 patients in the first year of treatment regardless of cause were 164.3, 194.5, and 206.4 for men receiving sipuleucel-T, enzalutamide, and abiraterone acetate, respectively. The average numbers of emergency department visits considered related to prostate cancer were 11.6, 16.0, and 14.1, respectively.
Survival Outcomes
Median overall survival in the overall analysis set was 22.97 months (Fig. 4). We compared the survival outcomes between treatments within the first-line cohort and within the any-line cohort, after controlling for the imbalances observed in the baseline populations (Table 2). Patients receiving sipuleucel-T as a first-line treatment had 44% reduction in the risk of death at 36 months compared to those receiving ASPIs as first-line treatment (adjusted HR, 0.56; 95% CI 0.494–0.627; P < 0.0001) (Table 2). Observed median overall survival was 34.9 months with first-line sipuleucel-T versus 21.0 months with first-line ASPIs–a 14-month difference in overall survival. A similar pattern of results was observed with the any-line cohort. There was a 41% decrease in the risk of death at 36 months in patients receiving sipuleucel-T vs. those receiving an oral ASPI (without sipuleucel-T) (adjusted HR, 0.59; 95% CI 0.527–0.651; P < 0.0001) (Table 2). This corresponded to an observed 14.5-month difference in median overall survival between the any-line groups, with durations of 35.2 months (sipuleucel-T) vs. 20.7 months (ASPI, without sipuleucel-T).
Kaplan-Meier Estimate of Overall Survival in the Overall Analysis Set. This graph illustrates the survival curve for men in the overall analysis set as described in Fig. 2a. This set includes 6044 men who were Medicare beneficiaries in 2014, who had a prostate cancer diagnosis for which they started treatment in that index year, and who received either sipuleucel-T or androgen-receptor signaling pathway inhibitors during the analysis period
Direct adjusted survival functions for both cohorts are illustrated in Fig. 5. Both curves exhibit consistent separation for the patients receiving sipuleucel-T and ASPIs, with the sipuleucel-T curves demonstrating improved survival at each time point.
Direct adjusted survivor functions based on the multivariable model by treatment. Panel a displays the results from the first-line cohort, and Panel b displays the results from the any-line cohort. Solid red lines reflect patients receiving sipuleucel-T as per cohort. Blue dashed lines reflect patients receiving androgen-receptor signaling pathway inhibitors (ASPIs) as per the indicated cohort. In the first-line cohort, patients could have received any agent in subsequent lines. In the any-line cohort, patients receiving ASPIs could not receive sipuleucel-T at any time during the observation period. In both cohorts, patients in the sipuleucel-T set exhibited improved overall survival compared to those in the ASPI set
Exploratory Analysis
No differences in overall survival were observed when we compared the sequence of first-line sipuleucel-T followed by ASPIs with first-line ASPIs followed by sipuleucel-T (HR, 1.17 [95% CI 0.72–1.91]; P = 0.521). Significantly better survival outcomes were observed when sipuleucel-T was used with an ASPI than either an ASPI alone or if two ASPIs were used in sequence (HR, 0.48 [95% CI 0.40–0.59]; P < 0.0001). Survival curves for these analyses are presented in Supplemental Figs. 2 and 3.
Discussion
Since the FDA approval of sipuleucel-T in 2010, there have been shifts in mCRPC treatment guidelines, and new treatment options have become available to clinicians for treating patients with advanced prostate cancer [3, 22,23,24,25,26,27,28,29,30,31]. However, little real-world evidence exists on the contemporary use and outcomes of these newer agents and sipuleucel-T consistent with their labeled indications at the time. Using the Medicare 100% dataset, a national longitudinal claims database linked to survival outcomes from a contemporary patient population, we used multivariate analysis to look at the relative benefits of treatment of advanced prostate cancer with sipuleucel-T and ASPIs in Medicare beneficiaries. There were small differences in the baseline population, including men receiving sipuleucel-T generally being slightly younger than those receiving ASPIs. Fewer men receiving sipuleucel-T were covered by both Medicare and Medicaid insurance (i.e., dual eligible), an indirect indicator of a lower socioeconomic status and more complex health needs. Overall, we found that after adjusting for baseline factors such as these, factors that may be confounders, the survival benefits of using sipuleucel-T and ASPIs in chemotherapy-naïve men with advanced prostate cancer remain apparent, with the risk of death dropping > 40% at 36 months regardless of whether used in the first line or in any line (Table 2, Figs. 4 and 5). While this information is important and hypothesis generating as we look to understand outcomes for patients with mCRPC, there are limitations and the need for validation across other datasets as well as trials to contextualize these results for clinical application.
The study on which the approval of sipuleucel-T is based, the phase III IMPACT trial, demonstrated that sipuleucel-T was associated with a prolonged median overall survival compared to placebo (25.8 months versus 21.7 months, P = 0.03) in men with a median baseline PSA of 51.7 ng/ml [25]. Post-hoc results of IMPACT also demonstrated that 3-year survival in the lowest baseline PSA quartile (< 22.1 ng/ml) was 62.6% for sipuleucel-T patients and 41.6% for control patients, representing a 50% relative increase [32]. Furthermore, results from the post-approval PROCEED registry (2011–2017) reported a median overall survival of 30.7 months in men with a median baseline PSA of 15 ng/ml, many of whom only received sipuleucel-T treatment [31], similar to the 35.2 months reported here with sipuleucel-T.
During the current study period, sipuleucel-T and ASPIs were indicated for use in men with asymptomatic or minimally symptomatic mCRPC without visceral metastases; we utilized multivariable modeling techniques to control for potential confounding variables and to minimize the risk of selection bias. The ‘risk’ variables included in our overall survival multivariable analysis (e.g., presence of multiple metastases, presence of SREs, Charlson Comorbidity Index score, chronic opiate use, and corticosteroid use) were all found to associate with decreased survival (Table 2)–providing internal validation that these covariates were prognostic factors of disease. Race was also a significant covariate, with black race being associated with improved outcomes, a finding consistent with previous studies [31,32,33]. Several key variables (e.g., cancer-related pain) are not available and have to be inferred on the basis of claims-level data (e.g., opiate usage). Given that clear assessment of symptoms was not present at baseline given the nature of the analysis, potential bias may exist as opiate use may not be a complete surrogate for baseline symptoms.
The Medicare 100% database also revealed significant variability in treatment patterns, with > 140 different patterns of care utilized in the study population [34]. These data highlight that mCRPC is an undertreated disease and that while six approved therapies were available in 2014, many patients only received one line of therapy (Fig. 3). Similar results were observed in the analysis of a contemporaneous patient population by George et al. [10]. Reasons for the limited use of available treatment options warrant further exploration including an explorations of patient preferences for sequencing treatments and the financial burden of sequencing multiple treatments.
Our initial results from a univariate analysis from the Medicare dataset described here suggested a 45% reduction in the risk of death and 14-month survival benefit with sipuleucel-T [15]. We expanded these analyses to explore outcomes using a multivariate analysis to evaluate the impact of sipuleucel-T on survival. Here, we focused on two comparisons: (1) the use of sipuleucel-T first line compared to the use of an ASPI first line, including men who received sipuleucel-T in the second-line or later; (2) the use of sipuleucel-T any line compared to an ASPI in any line without the use of sipuleucel-T. Both comparisons showed remarkably consistent results, with a similar magnitude of survival benefit observed after controlling for prognostic factors. This consistency suggests the impact of selection bias may be negligible.
Finally, an exploratory analysis assessing the clinical effects of sipuleucel-T when given sequentially or layered with an ASPI (sipuleucel-T in first-line or second-line) in the treatment paradigm revealed no difference in overall survival. Although this study was not designed to assess equivalency, we conducted exploratory analyses where we observed that inclusion of sipuleucel-T in first line or second line with an ASPI appeared to prolong survival in men with prostate cancer compared to using a single ASPI (alone) or a sequence of an ASPI followed by second ASPI. These findings support the need for further research to explore treatment sequences and therapeutic layering.
Of note, the current database includes minimal information on adverse events. Historically, the reported adverse events with sipuleucel-T have included short-lasting symptoms such as fever, headaches, chills, and myalgia, suggesting that it is well tolerated [35]. The PROCEED registry reported 13.7% of men had any serious adverse event and 2.8% had cerebrovascular events [31]. Dores et al. (2019) described adverse events reported in the FDA’s adverse event reporting system between April 29, 2010, and December 31, 2017, a timeframe that overlaps that of the current study [36]. Using this spontaneous safety surveillance database for drug and therapeutic biologic products, Dores et al. reported that events were generally consistent with those described in the prescribing information [36, 37]. When we look at our surrogate for serious adverse events, emergency department utilization for prostate cancer per 100 patients in the first year was 11.6, 16.0, and 14.1 for men receiving sipuleucel-T, enzalutamide, and abiraterone acetate, respectively.
The men described in the current retrospective study started their first treatment in 2014 and were followed for 36 months. Other descriptions of treatment outcomes in contemporaneous populations of men with mCRPC have been published recently: the observational PROCEED safety registry conducted in the US and described by Higano et al. [31], the analysis of electronic health records from US oncology practices curated by Flatiron and described by George et al. [10], and the report of a prospective ex-US patient registry described by Chowdhury et al. [38]. While not directly comparable, George et al. [10] and Higano et al. [31] may provide some clinical insight into the patients in the current study as they include contemporaneous and potentially overlapping populations. George et al. [10] reported a median PSA at diagnosis of 22.3 ng/ml, with median alkaline phosphatase, hemoglobin, and lactate dehydrogenase levels of as 98 U/l, 12.3 g/dl, and 197 U/l, respectively. Higano et al. [31] reported similar levels of median alkaline phosphatase, hemoglobin, and lactate dehydrogenase (82 U/l, 12.8 g/dl, and 186 U/l, respectively, albeit with a PSA of 15.0 ng/ml. Survival outcomes of George et al. [10] and the current study were similar: 22.97 months in our current study (Fig. 4) and 23.7 months (95% CI 22.3–25.1) in George et al. [10]. Higano et al. [31] reported longer survival outcomes (30.7 months) in its study of sipuleucel-T, possibly a reflection of the lower median PSA in that population [32]. We believe important insights can be drawn and together these real-world studies may paint a picture of treatment with the agents of interest in this setting. Data from the current study and both Higano et al. [31] and George et al. [10], all from the US, exhibit similar outcomes, likely a reflection of how available treatment guidelines inform physician prescribing practices. In contrast, Chowdhury et al. [38] describe treatment in Europe and other countries where sipuleucel-T is not available, and thus treatment guidelines differ. Furthermore, each study is represented by different types of data sources, each with their own set of research assumptions and biases.
The scope and quality of the Medicare 100% linked Part A, B, and D data from CMS provide a unique setting to facilitate research on a major medical condition in the Medicare population. We recognize that they do have significant limitations that restrict their direct clinical application [8, 9]. First, these data are at risk of selection bias and confounding by indication given that sipuleucel-T is approved for individuals with no to minimal symptoms without visceral metastases. By use of the multivariable modeling approach, we tested variables available for inclusion in the model and included significant ones. Second, the Medicare dataset lacks clinical information including factors known to be associated with survival (e.g., Eastern Cooperative Oncology Group performance status, lactate dehydrogenase, albumin, hemoglobin, prostate-specific antigen, and alkaline phosphatase) preventing us from being able to control for them [39]. Third, misclassification bias could also be present in an administrative claims database of this magnitude. Treating physicians must provide detailed and specific prior authorization factors to certify that the patient matches the labeled indication to obtain reimbursement for a Part-B drug (i.e., sipuleucel-T). This differs substantially from what is required to prescribe a Part-D drug like abiraterone or enzalutamide, which generally has fewer coding requirements to gain approval for use as treatment. As such, not all diagnostic coding fields need to be completed to prescribe Part-D drugs, potentially leading to an underreporting of metastatic status and other relevant covariates, such as sites of metastases. Unfortunately, this level of misclassification bias is outside our ability to control for in the regression modeling. Fifth, claims datasets such as this do not include safety assessments, aside from indirect ones based on claims, precluding their use in assessing safety. Finally, as this dataset predominately includes those 65 years and older, the outcomes observed may not be generalizable to younger patients. These limitations do not preclude the usefulness of these data, but they do indicate the importance of placing the study in the context of the available literature [10, 31].
Conclusion
The Medicare 100% dataset provides a unique opportunity to assess the real-world benefits of the standard drugs in our mCRPC arsenal. We demonstrate that the benefits of sipuleucel-T, which were first described a decade ago, still persist in the modern era of new treatments resulting in a median OS of 36 months regardless of line of use. After controlling for confounders such as baseline differences between groups by using a multivariable analysis, survival after sipuleucel-T treatment was significantly longer than that observed after ASPI treatment. Even given the potential limitations associated with claims analyses, such as selection bias and confounding by indication, this research provides important insights into real-world treatment outcomes and is complementary with other recently published real-world evidence analyses from other data sources [10, 31, 38]. In summary, this analysis provides important and hypothesis-generating information on the treatment of mCRPC that should be validated to contextualize these results for clinical application.
References
Schultz NM, Penson DF, Wilson SD, Song Y, Yang H, Ramaswamy K, et al. Health care resource utilization and costs associated with corticosteroid use in patients with castration-resistant prostate cancer: An administrative claims analysis. J Manag Care Spec Pharm. 2019;25(8):889–97.
Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65(11):1180–92.
Schweizer MT, Drake CG. Immunotherapy for prostate cancer: recent developments and future challenges. Cancer Metastasis Rev. 2014;33(2–3):641–55.
Madan RA, Antonarakis ES, Drake CG, Fong L, Yu EY, McNeel DG, et al. Putting the pieces together: completing the mechanism of action jigsaw for sipuleucel-T. J Natl Cancer Inst. 2020;112(6):562–73.
Caram MEV, Kaufman SR, Modi PK, Herrel L, Oerline M, Ross R, et al. Adoption of abiraterone and enzalutamide by urologists. Urology. 2019;131:176–83.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Prostate Cancer: version 1.2020, March 15, 2020: National Comprehensive Cancer Network; 2020 [NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines(R)) Prostate Cancer (Version 1.2020 March 15)]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
Pereira-Salgado A, Kwan EM, Tran B, Gibbs P, De Bono J, M IJ. Systematic review of efficacy and health economic implications of real-world treatment sequencing in prostate cancer: where do the newer agents enzalutamide and abiraterone fit in? Eur Urol Focus. 2020;ePub ahead of print.
Lyman GH. Comparative effectiveness research in oncology. Oncologist. 2013;18(6):752–9.
Di Maio M, Perrone F, Conte P. Real-world evidence in oncology: opportunities and limitations. Oncologist. 2020;25(5):e746–e752752.
George DJ, Sartor O, Miller K, Saad F, Tombal B, Kalinovsky J, et al. Treatment patterns and outcomes in patients with metastatic castration-resistant prostate cancer in a real-world clinical practice setting in the United States. Clin Genitourin Cancer. 2020;18(4):284–94.
Bynum J, Song Y, Fisher E. Variation in prostate-specific antigen screening in men aged 80 and older in fee-for-service Medicare. J Am Geriatr Soc. 2010;58(4):674–80.
Cohen JH, Schoenbach VJ, Kaufman JS, Talcott JA, Schenck AP, Peacock S, et al. Racial differences in clinical progression among Medicare recipients after treatment for localized prostate cancer (United States). Cancer Causes Control. 2006;17(6):803–11.
Cooper GS, Yuan Z, Jethva RN, Rimm AA. Determination of county-level prostate carcinoma incidence and detection rates with Medicare claims data. Cancer. 2001;92(1):102–9.
Sathiakumar N, Delzell E, Morrisey MA, Falkson C, Yong M, Chia V, et al. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis. 2011;14(2):177–83.
McKay R, Flanders SC, Ferro C, Fitch K, Fabrizio M, Schweizer M. Overall survival (OS) among Medicare beneficiaries receiving sipuleucel-T (SIP-T) vs oral treatment for metastatic castration resistant prostate cancer (mCRPC). J Clin Oncol. 2020;38(Suppl 6):42.
Halabi S, Lin C-Y, Kelly WK, Fizazi KS, Moul JW, Kaplan EB, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32(7):671–7.
Manitoba Centre for Health Policy. Concept: Charlson Comorbidity Index [website]. 2020 [updated 2020. Available from: https://mchp-appserv.cpe.umanitoba.ca/viewConcept.php?conceptID=1098.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9.
Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–9.
Hu Z-H, Peter Gale R, Zhang M-J. Direct adjusted survival and cumulative incidence curves for observational studies. Bone Marrow Transplant. 2020;55(3):538–43.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–33.
de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–54.
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005.
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–22.
Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–20.
Ryan CJ, Smith MR, Bono JS, Molina A, Logothetis CJ, Souza P. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48.
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12.
Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fossa SD. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23.
Higano CS, Armstrong AJ, Sartor AO, Vogelzang NJ, Kantoff PW, McLeod DG, et al. Real-world outcomes of sipuleucel-T treatment in PROCEED, a prospective registry of men with metastatic castration-resistant prostate cancer. Cancer. 2019;125:4172–80.
Schellhammer PF, Chodak G, Whitmore JB, Sims R, Frohlich MW, Kantoff PW. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology. 2013;81(6):1297–302.
Sartor O, Armstrong AJ, Ahaghotu C, McLeod DG, Cooperberg MR, Penson DF, et al. Survival of African–American and Caucasian men after sipuleucel-T immunotherapy: outcomes from the PROCEED registry. Prostate Cancer Prostatic Dis. 2020;23(3):517–26.
Flanders S, Bazell C, Ferro C, Fitch K, Hafron J, McKay R. Patterns of Drug Utilization for Metastatic Castration Resistant Prostate Cancer (mCRPC) Medicare Beneficiaries Receiving First-line Treatment 20th Annual Meeting of the Society of Urologic Oncology; Washington, DC2019. p. Abstract 192.
Higano CS, Small EJ, Schellhammer P, Yasothan U, Gubernick S, Kirkpatrick P, et al. Sipuleucel-T. Nat Rev Drug Discov. 2010;9(7):513–4.
Dores GM, Bryant-Genevier M, Perez-Vilar S. Adverse events associated with the use of sipuleucel-T reported to the US Food and Drug Administration’s adverse event reporting system, 2010–2017. JAMA Netw Open. 2019;2(8):e199249.
Dendreon Pharmaceuticals L. PROVENGE® (sipuleucel-T) Prescribing Information Seattle, WA2017 [Available from: https://www.provengehcp.com/Portals/5/Provenge-PI.pdf
Chowdhury S, Bjartell A, Lumen N, Maroto P, Paiss T, Gomez-Veiga F, et al. Real-world outcomes in first-line treatment of metastatic castration-resistant prostate cancer: the prostate cancer registry. Target Oncol. 2020;15(3):301–15.
Jarosek S. Death Information in the Research Identifiable Medicare Data 2018 [cited 2020 05/07/2020]. Available from: https://www.resdac.org/articles/death-information-research-identifiable-medicare-data.
Acknowledgements
Funding
Sponsorship for this study and the journal’s Rapid Service and Open Access fees was funded by Dendreon Pharmaceuticals, LLC.
Other Assistance
The authors thank Steven Metz of Milliman Inc. and Matthew Harmon, JD, of Dendreon Pharmaceuticals LLC for their biostatistical contributions.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentation
McKay R, Flanders SC, Ferro C, Fitch K, Fabrizio M, Schweizer M. Overall survival (OS) among Medicare beneficiaries receiving sipuleucel-T (sip-T) vs oral treatment for metastatic castration resistant prostate cancer (mCRPC). J Clin Oncol 2020;38(Suppl 6): Abstract 42. https://doi.org/10.1200/JCO.2020.38.6_suppl.42.
Disclosures
Dr. McKay reports research funding from Pfizer, Bayer, and Tempus; she also reports Advisory Board/Consulting for Bayer, Bristol Myers Squibb, Dendreon, Exelixis, Johnson and Johnson, Novartis, Pfizer Sanofi, and Vividion. Dr. Hafron reports personal fees from Amgen Inc, Bayer, and Blue Earth Diagnostics; grants and personal fees from Dendreon Pharmaceuticals LLC, Janssen Biotech Inc, Myriad Genetics Inc, Pfizer Inc, Astellas Pharma Inc, and Merck & Co. Inc.; and grants from Nucleix and Cellay Inc. outside the submitted work. Ms Ferro reports that her employer received fees from Dendreon Pharmaceuticals LLC, for data acquisition and analysis of data during the conduct of the study. Dr. Wilfehrt reports being an employee of Dendreon Pharmaceuticals, LLC during the conduct of the study and reports other for Amgen, CSL Ltd, and Illumina outside the submitted work. Ms Fitch reports that her employer received fees from Dendreon Pharmaceuticals LLC, for data acquisition and analysis of data during the conduct of the study. Dr. Flanders reports being an employee of Dendreon Pharmaceuticals, LLC during the conduct of the study and reports other from Johnson & Johnson, Inc, outside the submitted work. Dr. Fabrizio reports no conflicts of interest related to this work; however, he does report personal fees for advisory board participation and other for participation in a clinical research trial, both from Dendreon Pharmaceuticals, LLC. Dr. Schweizer reports personal fees from Janssen and his institution received research funds (other) from AstraZeneca, Pfizer, Madison Vaccines, and Hoffman-LaRoche outside the submitted work.
Compliance with Ethics Guidelines
This retrospective study used the secondary database, the Medicare 100% research dataset, which is based on anonymized patient claims data. Dendreon and Milliman had permission to access and use these data. This research is exempt from institutional review board approval.
Data Availability
Patient-level data remain in the possession of the Centers for Medicare and Medicaid Services and are not in the possession of either Dendreon or Milliman. Questions regarding the analysis methodology and outputs may be sent to mac@dendreon.com.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
McKay, R.R., Hafron, J.M., Ferro, C. et al. A Retrospective Observational Analysis of Overall Survival with Sipuleucel-T in Medicare Beneficiaries Treated for Advanced Prostate Cancer. Adv Ther 37, 4910–4929 (2020). https://doi.org/10.1007/s12325-020-01509-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01509-5