Abstract
Introduction
Allisartan isoproxil is a novel angiotensin II type 1 receptor antagonist that has been confirmed to lower blood pressure and protect target organs effectively. However, its role in improving endothelial function and vascular damage has not been investigated yet.
Methods
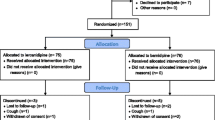
Patients with initially diagnosed mild essential hypertension (BP ranging from 140/90 to 159/99 mmHg) with age from 25–75 years were randomly assigned 1:1 to either the allisartan group (allisartan 240 mg/day and lifestyle modification) or the lifestyle modification group and were followed up for 30 days. Flow-mediated dilation (FMD), brachial-ankle pulse wave velocity (baPWV) and endothelial microparticles (EMPs) were measured for evaluation of endothelial function and vascular damage. In addition, we enrolled 36 normotensive individuals as healthy control.
Results
Seventy-two mildly hypertensive patients were enrolled in this study. After 30 days of treatment, a significant increase in FMD was observed in the allisartan group (0.9 ± 0.7%, p < 0.001) and remained unchanged in the lifestyle modification group, but the difference between the two groups did not reach statistical significance (p = ns). EMPs, baPWV, SBP and DBP decreased by 251.0 ± 255.9 counts/μl (p < 0.001), 102.8 ± 84.2 cm/s (p < 0.001), 13.20 ± 3.9 mmHg (p < 0.001) and 9.35 ± 2.5 mmHg (p < 0.001), respectively, in the allisartan group, while by 21.3 ± 84.3 counts/μl (p = ns), 0.4 ± 22.0 cm/s (p = ns), 3.2 ± 6.0 mmHg (p < 0.01) and 1.0 ± 2.5 mmHg (p = ns), respectively, in the lifestyle modification group. All of the indexes above achieved statistical significance between the allisartan and lifestyle modification groups (p < 0.05). Besides, after 30 days of allisartan administration baPWV and EMPs were comparable to those measured in the healthy control group, while the difference in SBP, DBP and FMD remained significant between the allisartan and healthy control groups (p < 0.05).
Conclusion
The present study demonstrates for the first time that allisartan isoproxil exerts a favorable effect on improving endothelial function and vascular damage in patients with mild EH, making it a promising drug for management of EH.
Clinical Trial Registration
ChiCTR2000032332.





Similar content being viewed by others
References
Hong KN, Fuster V, Rosenson RS, Rosendorff C, Bhatt DL. How low to go with glucose, cholesterol, and blood pressure in primary prevention of CVD. J Am Coll Cardiol. 2017;70(17):2171–85.
Alexander RW. Theodore Cooper Memorial Lecture. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995;25(2):155–61.
Deferrari G, Ravera M, Deferrari L, Vettoretti S, Ratto E, Parodi D. Renal and cardiovascular protection in type 2 diabetes mellitus: angiotensin II receptor blockers. J Am Soc Nephrol. 2002;13(Suppl 3):S224–S22929.
Koh KK, Han SH, Chung WJ, et al. Comparison of effects of losartan, irbesartan, and candesartan on flow-mediated brachial artery dilation and on inflammatory and thrombolytic markers in patients with systemic hypertension. Am J Cardiol. 2004;93(11):1432–5 (A10).
Perrone-Filardi P, Corrado L, Brevetti G, et al. Effects of AT1 receptor antagonism with candesartan on endothelial function in patients with hypertension and coronary artery disease. J Clin Hypertens (Greenwich). 2009;11(5):260–5.
Koh KK, Quon MJ, Han SH, et al. Distinct vascular and metabolic effects of different classes of anti-hypertensive drugs. Int J Cardiol. 2010;140(1):73–81.
Li S, Wu Y, Yu G, Xia Q, Xu Y. Angiotensin II receptor blockers improve peripheral endothelial function: a meta-analysis of randomized controlled trials. PLoS ONE. 2014;9(3):e90217.
Oparil S, Williams D, Chrysant SG, Marbury TC, Neutel J. Comparative efficacy of olmesartan, losartan, valsartan, and irbesartan in the control of essential hypertension. J Clin Hypertens (Greenwich). 2001;3(5):283–91.
Li Y, Li XH, Huang ZJ, et al. A randomized, double blind, placebo-controlled, multicenter phase II trial of allisartan isoproxil in essential hypertensive population at low-medium risk. PLoS ONE. 2015;10(2):e0117560.
Zhang JQ, Yang GH, Zhou X, et al. Effects of allisartan isoproxil on blood pressure and target organ injury in patients with mild to moderate essential hypertension. Medicine (Baltimore). 2019;98(12):e14907.
Derosa G, Cicero AF, Bertone G, et al. Comparison of the effects of telmisartan and nifedipine gastrointestinal therapeutic system on blood pressure control, glucose metabolism, and the lipid profile in patients with type 2 diabetes mellitus and mild hypertension: a 12-month, randomized double-blind study. Clin Ther. 2004;26(8):1228–366.
Thijssen DHJ, Bruno RM, van Mil A, et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J. 2019;40(30):2534–47.
Munakata M. Utility of automated brachial ankle pulse wave velocity measurements in hypertensive patients. Am J Hypertens. 2003;16(8):653–7.
Wang JM, Su C, Wang Y, et al. Elevated circulating endothelial microparticles and brachial-ankle pulse wave velocity in well-controlled hypertensive patients. J Hum Hypertens. 2009;23(5):307–15.
Wang J, Huang Y, Wang Y, et al. Increased circulating CD31+/CD42− microparticles are associated with impaired systemic artery elasticity in healthy subjects. Am J Hypertens. 2007;20(9):957–64.
Cheng F, Wang Y, Li J, et al. Berberine improves endothelial function by reducing endothelial microparticles-mediated oxidative stress in humans. Int J Cardiol. 2013;167(3):936–42.
Schiro A, Wilkinson FL, Weston R, Smyth JV, Serracino-Inglott F, Alexander MY. Endothelial microparticles as conveyors of information in atherosclerotic disease. Atherosclerosis. 2014;234(2):295–302.
Romero CA, Orias M, Weir MR. Novel RAAS agonists and antagonists: clinical applications and controversies. Nat Rev Endocrinol. 2015;11(4):242–52.
Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure-lowering on outcome incidence in hypertension:5. Head-to-head comparisons of various classes of antihypertensive drugs—overview and meta-analyses. J Hypertens. 2015;33:1321–41.
Thomopoulos C, Parati G, Zanchetti A. Effects of blood-pressure-lowering treatment on outcome incidence. 12. Effects in individuals with high-normal and normal blood pressure: overview and meta-analyses of randomized trials. J Hypertens. 2017;35(11):2150–60.
Wu MY, Ma XJ, Yang C, et al. Effects of allisartan, a new AT(1) receptor blocker, on blood pressure and end-organ damage in hypertensive animals. Acta Pharmacol Sin. 2009;30(3):307–13.
Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1990s;362(6423):801–9.
Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340(2):115–26.
Zhou QB, Xia WH, Ren J, et al. Effect of intensive periodontal therapy on blood pressure and endothelial microparticles in patients with prehypertension and periodontitis: a randomized controlled trial. J Periodontol. 2017;88(8):711–22.
Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236–41.
Tropeano AI, Boutouyrie P, Pannier B, et al. Brachial pressure-independent reduction in carotid stiffness after long-term angiotensin-converting enzyme inhibition in diabetic hypertensives. Hypertension. 2006;48(1):80–6.
Cohn JN, Duprez DA, Grandits GA. Arterial elasticity as part of a comprehensive assessment of cardiovascular risk and drug treatment. Hypertension. 2005;46(1):217–20.
Jain M, Bhosale V, Tripathi D, et al. Antihypertensive drugs aliskiren, nebivolol, and olmesartan reduce hypertension by reducing endothelial microparticles and regulating angiogenesis. J Cardiovasc Pharmacol. 2017;70(3):176–83.
Nomura S, Shouzu A, Omoto S, Nishikawa M, Fukuhara S, Iwasaka T. Losartan and simvastatin inhibit platelet activation in hypertensive patients. J Thromb Thrombolysis. 2004;18(3):177–85.
Acknowledgements
We appreciate the patients and healthy volunteers for participating in this study.
Funding
This work was supported by the National Nature Science Foundation (31530023) of China, 973 Program (2013CB531200), and the Science and Technology Planning Project (201704020212) of Guangzhou. The journal's rapid service fee was supported by the National Nature Science Foundation (31530023) of China.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, contributed to the writing and reviewing of the manuscript, and have given final approval for the version to be published.
Author Contributions
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. Yongqiang Fan, Yumin Qiu, Zhe Zhou, Zhichao Wang, Yuanya Liu, and Xing Liu were involved in the acquisition of data. Gaoxing Zhang and Jun Tao were involved in the conception and design of the study. Jianning Zhang was involved in the data analysis.
Disclosures
Gaoxing Zhang, Yongqiang Fan, Yumin Qiu, Zhe Zhou, Jianning Zhang, Zhichao Wang, Yuanya Liu, Xing Liu and Jun Tao have nothing to disclose.
Compliance with Ethics Guidelines
The study was performed in accordance with International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use good clinical practice guidelines and the principles of the Declaration of Helsinki. The protocol was approved by all appropriate institutional review boards or independent ethics committees [The First Affiliated Hospital, Sun Yat-Sen University (Guangzhou, China)]. All patients gave informed consent before study participation.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital features To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12471605.
Rights and permissions
About this article
Cite this article
Zhang, G., Fan, Y., Qiu, Y. et al. Allisartan Isoproxil Improves Endothelial Function and Vascular Damage in Patients with Essential Hypertension: A Single-Center, Open-Label, Randomized Controlled Trial. Adv Ther 37, 3551–3561 (2020). https://doi.org/10.1007/s12325-020-01413-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01413-y