Abstract
Introduction
To optimize the aflibercept treat-and-extend protocol in wet age-related macular degeneration (wAMD) beyond the 1-year interim report.
Methods
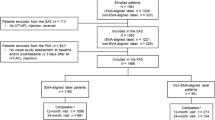
This 2-year prospective randomized clinical trial included 52 eyes from 52 patients with treatment-naïve wAMD. After the induction phase of three monthly aflibercept injections, patients were randomized 1:1 to two different treat-and-extend protocols. In the treat-and-extend protocol with moderate extensions (T&Em), the treatment interval was extended 1 week at a time up to 12 weeks, and then by 2 weeks up to 16 weeks. In the treat-and-extend protocol with rapid extensions (T&Er), the treatment interval was initially extended to 8 weeks, and then by 2 weeks up to 16 weeks. Main outcome measure was the number of given aflibercept injections.
Results
At the study end point at 2 years, the mean visual gain from the baseline was 7.9 ± 14.5 letters in T&Em, compared to 10.8 ± 16.5 letters in T&Er protocol (P = 0.726). The mean decrease in central subfield macular thickness was 203.0 ± 167.4 µm in T&Em and 192.3 ± 160.2 µm in T&Er protocol (P = 0.822). Treatment interval was 10.3 ± 3.3 weeks in T&Em and 11.7 ± 3.5 in T&Er protocol (P = 0.164) at the end of year 2. The total number of injections in 2 years was 14.1 ± 3.1 in T&Em and 11.6 ± 2.0 in T&Er (P = 0.002), and the number of injections during the second year was 5.4 ± 1.8 and 4.4 ± 1.4, respectively (P = 0.043). A total of 71% of the eyes in both treatment groups had a dry macula at the study end point.
Conclusions
At 2 years, the anatomical and functional responses between the two treatment groups were similar. However, the number of given aflibercept injections was smaller in the rapid extensions protocol.
Trial Registration
EU Clinical Trials Register Number, 2015-001394-41/FI



Similar content being viewed by others

References
Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Global Health. 2017;5(12):e1221–e12341234.
Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358(24):2606–17.
Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728–38.
Bloch SB, Larsen M, Munch IC. Incidence of legal blindness from age-related macular degeneration in denmark: year 2000 to 2010. Am J Ophthalmol. 2012;153(2):209–13.e2.
Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–98.
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57–65.e5.
Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–31.
Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–48.
Spaide RF. The as-needed treatment strategy for choroidal neovascularization: a feedback-based treatment system. Am J Ophthalmol. 2009;148(1):1–3.
Berg K, Hadzalic E, Gjertsen I, et al. Ranibizumab or bevacizumab for neovascular age-related macular degeneration according to the lucentis compared to avastin study treat-and-extend protocol: two-year results. Ophthalmology. 2016;123(1):51–9.
Wykoff CC, Ou WC, Brown DM, et al. Randomized trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: 2-year results of the TREX-AMD study. Ophthalmo Retina. 2017;1(4):314–21.
Haga A, Kawaji T, Ideta R, Inomata Y, Tanihara H. Treat-and-extend versus every-other-month regimens with aflibercept in age-related macular degeneration. Acta Ophthalmol. 2018;96(3):e393–e398398.
Taipale C, Lindholm JM, Laine I, Tuuminen R. Comparison of two different treat-and-extend protocols with aflibercept in wet age-related macular degeneration. Acta Ophthalmol. 2019. https://doi.org/10.1111/aos.14231.
DeCroos FC, Reed D, Adam MK, et al. Treat-and-extend therapy using aflibercept for neovascular age-related macular degeneration: a prospective clinical trial. Am J Ophthalmol. 2017;180:142–50.
Ohji M, Takahashi K, Okada AA, et al. Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-week findings from ALTAIR: a randomized controlled trial. Adv Ther. 2020;37(3):1173–87.
Tuuminen R, Uusitalo-Jarvinen H, Aaltonen V, et al. The Finnish national guideline for diagnosis, treatment and follow-up of patients with wet age-related macular degeneration. Acta Ophthalmol. 2017;95(A105 Suppl):1–9.
Chakravarthy U, Harding SP, Rogers CA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382(9900):1258–67.
Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Global Health. 2014;2(2):e106–e116116.
Tuulonen A, Kataja M, Syvanen U, Miettunen S, Uusitalo H. Right services to right patients at right time in right setting in Tays Eye Centre. Acta Ophthalmol. 2016;94(7):730–5.
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121(1):193–201.
Nguyen V, Daien V, Guymer R, et al. Projection of long-term visual acuity outcomes based on initial treatment response in neovascular age-related macular degeneration. Ophthalmology. 2019;126(1):64–74.
Berg K, Pedersen TR, Sandvik L, Bragadottir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology. 2015;122(1):146–52.
Silva R, Berta A, Larsen M, Macfadden W, Feller C, Mones J. Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the TREND study. Ophthalmology. 2018;125(1):57–655.
Guymer RH, Markey CM, McAllister IL, et al. Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: FLUID study 24-month results. Ophthalmology. 2019;126(5):723–34.
Acknowledgements
We thank Ms. Reetta Österberg and Ms. Enni Särkkä for their valuable work as research assistants. We thank the participants of the study.
Funding
The study was supported by grants from the Finnish Eye Foundation, Finnish Ophthalmological Society, the Nissi Foundation, Orion Research Foundation, the Paulo Foundation, the Waldemar von Frenckell Foundation, Glaukooma tukisäätiö LUX, and the HUS Specific Catchment Area (ERVA) Clinical Research Grants. The Rapid Service Fees were covered by Bayer Pharma AG.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Claudia Taipale, Juha-Matti Lindholm, Kai Kaarniranta, and Raimo Tuuminen have nothing to disclose.
Compliance with Ethics Guidelines
The approvals of the Research Director and the Chief Medical Officer of Kymenlaakso Central Hospital, the Finnish Medicines Agency Fimea, and the Institutional Review Board of Helsinki University Hospital were obtained. The study followed the tenets of the Declaration of Helsinki. A written consent was received from all patients before enrollment. Bayer Pharma AG supplied the intravitreal aflibercept injections used in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12006759.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Taipale, C., Lindholm, JM., Kaarniranta, K. et al. Comparison of Two Different Treat-and-Extend Protocols with Aflibercept in Wet Age-Related Macular Degeneration: Two-Year Results. Adv Ther 37, 2256–2266 (2020). https://doi.org/10.1007/s12325-020-01312-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01312-2