Abstract
Introduction
The present study aimed to indirectly compare apalutamide and enzalutamide with respect to tolerability and health-related quality of life (HRQoL) among men with non-metastatic castration-resistant prostate cancer (nmCRPC).
Methods
Patient-level data from the SPARTAN study [apalutamide + androgen deprivation therapy (ADT) versus placebo + ADT] and aggregate published data from the PROSPER study (enzalutamide + ADT versus placebo + ADT) were used. Anchored matching-adjusted indirect comparison (MAIC) was conducted by weighting patients’ baseline characteristics from SPARTAN to match aggregated baseline characteristics in PROSPER. Odds ratios (ORs) of reported adverse events (AEs) and baseline-to-follow-up least squares mean differences in HRQoL [measured with Functional Assessment of Cancer Therapy-Prostate (FACT-P) score] with 95% credible intervals were re-estimated for SPARTAN arms using weighted population and indirectly compared with those in PROSPER through a Bayesian framework. Events of special interest included fatigue, hot flush, nausea, diarrhea, hypertension, falls, dizziness, decreased appetite, arthralgia, asthenia and headache. In addition, any AEs and serious AEs were explored.
Results
Of 1207 SPARTAN patients, 1171 were matched to 1401 PROSPER patients. Relative to enzalutamide, apalutamide demonstrated better tolerability as evidenced by the highest probability of reduced occurrence of fatigue [p(OR < 1) = 99.5%], hypertension [p(OR < 1) = 99.2%], decreased appetite [p(OR < 1) = 98.3%], fall [p(OR < 1) = 90.3%], headaches [p(OR < 1) = 86.7%], and nausea [p(OR < 1) = 80.0%]. The probabilities of reduced occurrence of any AEs and SAEs with apalutamide versus enzalutamide were 66.9% and 90.9%, respectively. Relative to enzalutamide, apalutamide treatment was associated with a higher probability of a better HRQoL based on the FACT-P total score [p(diff > 0) = 73.1%]. The probability of a better HRQoL with apalutamide versus enzalutamide was highest for the physical [p(diff > 0) = 97.3%] and functional [p(diff > 0) = 86.7%] wellbeing subscales, and the pain-related subscale [p(diff > 0) = 90.1%].
Conclusion
Anchored MAIC suggests that treatment of men with nmCRPC with apalutamide is associated with a higher probability of better tolerability due to fewer AEs and better HRQoL than enzalutamide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
In the absence of clinical studies directly comparing apalutamide and enzalutamide with respect to adverse events (AEs) and health-related quality of life (HRQoL), an anchored matching-adjusted indirect comparison was conducted to inform treatment-related decision-making in non-metastatic castration-resistant prostate cancer (nmCRPC). |
What did the study ask? |
Considerations for optimizing treatment tolerability and maintaining a good HRQoL while delaying progression are relevant treatment goals for patients with nmCRPC. Therefore, this analysis assessed which treatment has the higher probability of better tolerability and which has the higher probability of better HRQoL. |
What was learned from the study? |
The probability of improved tolerability with apalutamide versus enzalutamide exceeded 80%, as evidenced by reduced occurrence of fatigue, hypertension, decreased appetite, fall, headaches, and serious AEs. Treatment with apalutamide was associated with a higher probability of better HRQoL compared with enzalutamide. |
Introduction
Non-metastatic castration-resistant prostate cancer (nmCRPC) is associated with a poor prognosis, with a median metastasis-free survival (MFS) reaching approximately 15 months when treated with androgen deprivation therapy (ADT) alone [1, 2]. Continuous ADT is advised for nmCRPC until it progresses to metastatic disease [3]. The androgen receptor inhibitors apalutamide and enzalutamide became the first treatments approved by the US Food and Drug Administration and European Medicines Agency for nmCRPC [4,5,6,7], based on results from the phase III, randomized SPARTAN and PROSPER studies, respectively [1, 2]. The National Comprehensive Cancer Network, the American Urological Association, and the European Association of Urology recommend that patients with nmCRPC with a prostate-specific antigen doubling time (PSADT) of ≤ 10 months be treated with apalutamide or enzalutamide plus continuous ADT [3, 8, 9]; French guidelines recommend both therapies regardless of the risk of progression [10].
In SPARTAN, MFS was significantly improved with apalutamide + ADT versus placebo + ADT (hereafter referred to as the apalutamide and ADT arms, respectively) in patients with nmCRPC [2]. Similarly, enzalutamide + ADT significantly improved MFS relative to placebo + ADT (hereafter referred to as the enzalutamide and ADT arms, respectively) in PROSPER [1].
The efficacy and tolerability of apalutamide and enzalutamide have not been directly compared in a randomized controlled study. Two previous studies concluded that both drugs probably have similar efficacy and tolerability profiles by indirectly comparing the results of the SPARTAN and PROSPER studies using a method called the Bucher technique [12, 13]. This method relies solely on comparisons of aggregate data between studies without considering differences in baseline characteristics [14]. However, results from a recent matching-adjusted indirect comparison (MAIC) study suggested that nmCRPC patients treated with apalutamide have a higher probability of more favorable MFS and overall survival than patients treated with enzalutamide [11]. Anchored MAIC may overcome potential biases introduced by imbalances in patient characteristics by reweighting individual patient data from one study so that measured baseline characteristics match those in the study of the comparator treatment [15,16,17].
With new treatments changing the prognosis for patients with nmCRPC, health-related quality of life (HRQoL) becomes an important performance measure in addition to survival [18, 19]. Reflecting a patient-centric approach to healthcare interventions, HRQoL provides important insights into the impact of a treatment on patients’ daily lives, which can help patients and clinicians make more informed treatment decisions that may improve the patient experience [20, 21]. For instance, given the pain associated with bone metastases upon progression to metastatic disease [22], choosing a treatment that will maintain a stable HRQoL while delaying the onset of metastases is highly relevant for patients with nmCRPC.
Both the SPARTAN and PROSPER studies assessed HRQoL outcomes. In SPARTAN, a least squares (LS) mean change from baseline showed an HRQoL deterioration that was numerically more apparent in the ADT arm than in the apalutamide arm [23]. In PROSPER, Tombal et al. reported that HRQoL deteriorated significantly less rapidly in the enzalutamide arm than in the ADT arm [24]. However, HRQoL outcomes have to date not been compared between apalutamide and enzalutamide. Using an anchored MAIC, this study aimed to compare apalutamide and enzalutamide with respect to tolerability and HRQoL.
Methods
Data Source
Individual patient data from SPARTAN [2, 23] and aggregate data from PROSPER [1, 25] were used. The SPARTAN intention-to-treat (ITT) population included 1207 patients (apalutamide: 806; ADT: 401) [2]. The PROSPER ITT population included 1401 patients (enzalutamide: 933; ADT: 468) [1]. AE and HRQoL analyses were based on the ITT populations from the two studies and included patients with available baseline and follow-up measurements. Review boards at participating institutions approved the SPARTAN and PROSPER studies, and they were conducted in accordance with the current International Conference on Harmonisation guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki.
Endpoints
The following AEs, reported in both studies, and of special interest as they are included within regulatory summaries, were compared: any AEs, serious AEs (SAEs), fatigue, hot flush, nausea, diarrhea, hypertension, fall, dizziness, decreased appetite, arthralgia, asthenia, and headache. SAEs were events that resulted in death, were life-threatening, resulted in or prolonged hospitalization, resulted in inability to conduct normal life functions, or led to a congenital anomaly or birth defect.
HRQoL was compared using Functional Assessment of Cancer Therapy-Prostate (FACT-P) data available in both studies. FACT-P is a disease-specific and validated instrument to measure HRQoL in patients with prostate cancer [26]. This validated questionnaire includes the original subscales of the 27-item FACT-general (FACT-G) [27] supplemented by a 12-item prostate cancer-specific subscale (PCS) that aims to assess potential issues with sexuality, bowel/bladder function, and pain [26, 28]. The following subscales are assessed with this instrument: physical wellbeing (PWB), social wellbeing, emotional wellbeing, functional wellbeing (FWB), and PCS. Other composite scores can also be derived from the FACT-P, including the Prostate Cancer Pain-related score (PCPS; three pain-related questions from the PCS and one pain item from the PWB subscale), the FACT Advanced Prostate Symptom Index, and the Trial Outcome Index (PWB, FWB, and the PCS scales) [29, 30]. Subscale scores can be added together to make a single overall score (i.e., FACT-P total score), which ranges from 0 to 156. Higher values of FACT-P (total and any subscale) indicate better HRQoL.
In SPARTAN, HRQoL was evaluated at each cycle up to cycle 7 (one cycle = 28 days), every two cycles from cycle 7 to cycle 13, and every four cycles for cycles 13–29; AEs were evaluated every cycle [2]. In PROSPER, HRQoL and AEs were evaluated every four cycles [25]. In the current study, baseline and follow-up (at week 96 for SPARTAN and at week 97 for PROSPER, the closest time points available for comparison) measurements of HRQoL were used.
Statistical Analyses
The algorithm was the same as that used in a previous anchored MAIC study that compared the efficacy of apalutamide and enzalutamide in patients with nmCRPC [11].
Step 1: Recalculation of Proportions and Least Squares Mean Differences from SPARTAN
The baseline characteristics of patients enrolled in SPARTAN were matched to those of patients enrolled in PROSPER via inverse probability of treatment weighting. The propensity score model was estimated using the generalized method of moments [31]. All clinically relevant baseline characteristics reported in PROSPER that could potentially affect the relative treatment effect were considered in the matching process: baseline PSA and PSADT, Eastern Cooperative Oncology Group performance status, total Gleason score, baseline use of bone-targeting agents, and baseline history of surgical prostate cancer procedures. Patients from SPARTAN missing any of the matched characteristics were excluded from the sample. Weighted odds ratios (ORs) were estimated to compare AEs for apalutamide versus ADT based on MAIC-weighted data from SPARTAN. Weighted LS mean differences with 95% confidence intervals (CIs) were estimated to compare baseline-to-follow-up change in FACT-P scores (total and subscales) between apalutamide and ADT using MAIC-weighted data from SPARTAN. This process was performed at a study level, thereby preserving the randomization of patients in the original studies.
Step 2: Bayesian Network Meta-analysis
Bayesian network meta-analysis with non-informative prior distributions was used to indirectly compare both treatments with respect to AEs and HRQoL [16, 32]. The ORs (for AEs) and LS mean differences (for HRQoL) of the reweighted SPARTAN population from step 1 were compared with those reported in PROSPER to estimate ORs and LS mean differences for apalutamide versus enzalutamide, with ADT as the common comparator for both studies. Posterior distributions, including 95% credible intervals (CrIs) and the probability of apalutamide being better than enzalutamide, were reported. All analyses were conducted according to the methods described in the National Institute for Health and Care Excellence Decision Support Unit Technical Support Documents [33, 34]. Statistical significance is a frequentist concept and should not be applied within the Bayesian framework; therefore, the focus is placed on the probability of OR < 1 to assess the likelihood that one treatment is better than the other, wherever the Bayesian results are presented.
Results
Baseline Characteristics and Matching
Baseline characteristics of patients enrolled in SPARTAN before and after matching have been reported in a previous MAIC study that compared the efficacy of apalutamide and enzalutamide [11]. Prior to matching, patient populations differed with respect to median PSADT (SPARTAN: 4.4 months; PROSPER: 3.7 months), proportion of patients with PSADT < 6 months (SPARTAN: 70%; PROSPER: 77%), and median serum PSA levels (SPARTAN: 7.8 ng/ml; PROSPER: 10.8 ng/ml). The proportion of patients using bone-targeting agents was 10% in SPARTAN and 11% in PROSPER. A total of 36 patients from SPARTAN with missing information for matched variables were excluded (Table 1). After matching, baseline characteristics of patients from both studies were balanced.
Adverse Events
In the apalutamide arm of the SPARTAN study, 96.5% of patients had AEs before matching; this proportion was 96.3% after matching (Table 2). In the ADT arm of SPARTAN, 93.2% and 94.0% had AEs before and after matching, respectively (Table 2). Among patients in the apalutamide arm in SPARTAN, 24.8% and 23.7% had SAEs before and after matching, respectively. In the ADT arm, these proportions were 23.1% and 22.3%, respectively (Table 2).
Apalutamide had a higher probability of better tolerability versus enzalutamide as evidenced by reduced occurrence of the following events: fatigue (OR [95% CrI] 0.57 [0.37; 0.88], p[OR < 1] = 99.5%), hypertension (OR [95% CrI] 0.50 [0.29; 0.87], p[OR < 1] = 99.2%), decreased appetite (OR [95% CrI] 0.48 [0.25; 0.95], p[OR < 1] = 98.3%), fall (OR [95% CrI] 0.65 [0.34; 1.25], p[OR < 1] = 90.3%), headache (OR [95% CrI] 0.68 [0.34; 1.36], p[OR < 1] = 86.7%), and nausea (OR [95% CrI] 0.80 [0.48; 1.35], p[OR < 1] = 80.0%) (Fig. 1). Conversely, the probabilities of occurrence of diarrhea (OR [95% CrI] 1.57 [0.94; 2.62], p[OR < 1] = 4.3%) and arthralgia (OR [95% CrI] 1.91 [1.04; 3.53], p[OR < 1] = 1.8%) were higher with apalutamide versus enzalutamide. The probability that patients treated with apalutamide would have better tolerability versus enzalutamide based on overall lower rates of any AEs was 66.9% (OR [95% CrI] 0.86 [0.44; 1.67]). Importantly, the probability that patients treated with apalutamide would have overall lower rates of any SAEs versus enzalutamide was 90.9% (OR [95% CrI] 0.76 [0.50; 1.14]).
Health-Related Quality of Life
In SPARTAN, the baseline-to-follow-up improvement in FACT-P was numerically more in favor of apalutamide versus ADT after matching (LS mean difference [95% confidence interval (CI)] 3.53 [-0.18; 7.24]) versus before matching (LS mean difference [95% CI] 3.34 [− 0.23; 6.91]; Table 3). Using matched data, the MAIC results suggested a 73.1% probability of a more favorable improvement in FACT-P total score with apalutamide versus enzalutamide (LS mean difference [95% CrI] 1.50 [− 3.27; 6.27]; Table 4 and Fig. 2). The probabilities of a more favorable improvement in the PWB (LS mean difference [95% CrI] 1.12 [− 0.01; 2.25], p[diff > 0] = 97.3%) and FWB (LS mean difference [95% CrI] 0.85 [− 0.64; 2.34], p[diff > 0] = 86.7%) subscale scores with apalutamide versus enzalutamide were particularly high (Table 4 and Fig. 2). The probability of a more favorable improvement in PCSP with apalutamide versus enzalutamide also appeared pronounced (LS mean difference [95% CrI] 0.63 [− 0.33; 1.59], p[diff > 0] = 90.1%; Table 4 and Fig. 2). Similar trends were observed for the other scores, subscales, or composite scores assessed (Table 4 and Fig. 2).
Discussion
Using an anchored MAIC, the present study indirectly compared tolerability and HRQoL among men with high-risk nmCRPC who, in the framework of two independent clinical studies, received apalutamide (SPARTAN) and enzalutamide (PROSPER). The probabilities of apalutamide having a more improved tolerability profile as evidenced by the reduced occurrence of several specific AEs, including fatigue, decreased appetite, hypertension, and fall, versus enzalutamide exceeded 90%. Additionally, this MAIC demonstrated, with high probability, that apalutamide had a more favorable tolerability as defined by reduced occurrence of any AE (66.9% probability) or a reduced occurrence of SAEs (90.9% probability) than enzalutamide. The probability of a better HRQoL measured based on a change in the FACT-P total score was 73.1% with apalutamide versus enzalutamide. The probabilities of a more favorable change in all FACT-P subscale scores with apalutamide versus enzalutamide were also pronounced. Taken together, these results suggest that men with nmCRPC treated with apalutamide are more likely to experience fewer AEs and better HRQoL than men treated with enzalutamide.
In the present study, the probability of fewer AEs (any) with apalutamide relative to enzalutamide reached 66.9%. This probability was higher for SAEs (90.9%). This is notable because the study design of SPARTAN allowed for more frequent AE reporting (every 4 weeks) than in PROSPER (every 16 weeks). Despite the more frequent collection of AE events in SPARTAN, which would theoretically increase the probability of detecting more AEs, the results of this study support higher probability of overall lower AEs with apalutamide treatment, suggesting that the tolerability profile of apalutamide could be better than enzalutamide. With regard to the individual AEs assessed, fatigue was most differentiated between apalutamide and enzalutamide. Management of fatigue may be an important factor for overall treatment success in patients with advanced prostate cancer. For example, in the PREVAIL study of enzalutamide in metastatic CRPC, fatigue was the most common AE leading to treatment discontinuation [35]. Although additional research is needed to validate the relationship of fatigue-related treatment discontinuation in nmCRPC, the observation in this study of a higher probability of reduced fatigue observed with apalutamide versus enzalutamide could be an important consideration for treatment optimization.
Another noteworthy observation was the high probability of reduced occurrence of dizziness and falls among apalutamide users compared with enzalutamide users. These two AEs are likely associated with an increased risk of accidental fracture. While both agents delay pathologic fractures secondary to metastases through their antitumor activity [1, 2], they are used concomitantly with ADT, which is known to be associated with bone mineral density loss and increased risk of accidental fracture [36]. Therefore, the high probability of reduced occurrence of dizziness and falls with apalutamide relative to enzalutamide may also lead to a reduced incidence of accidental fractures. In contrast, the higher probability of occurrence of diarrhea with apalutamide versus enzalutamide might be related to differences in the formulation of the two agents. At the start of the SPARTAN study, patients were initiated on an apalutamide formulation in soft-gel capsules, but these were subsequently changed to tablets after a protocol amendment. Data collected from the SPARTAN study indicate that patients receiving capsules experienced more AEs, including GI-related AEs, than patients receiving tablets. Given that excipients may cause gastrointestinal side effects, it is unclear how the initial formulation of apalutamide affected the incidence of diarrhea.
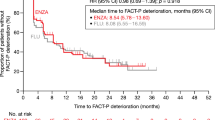
Saad et al. recently published the results of a prespecified exploratory analysis of HRQoL in SPARTAN, that was assessed with FACT-P [23]. In the analysis of time to deterioration of the FACT-P score, there was no difference between the study arms based on the CIs. When evaluating LS mean changes during follow-up versus baseline, a deterioration of HRQoL was numerically more apparent in the ADT arm than in the apalutamide arm. In PROSPER, discrepancies in the time to FACT-P deterioration exist between the unconfirmed and confirmed (i.e., deterioration confirmed at the next consecutive visit) analyses [1, 24]. In the confirmed analysis, a significantly less rapid deterioration was observed in the enzalutamide arm compared with the ADT arm [24]. In terms of the LS mean changes in the FACT-P score during follow-up versus baseline, a numerical but not statistical advantage was observed for the enzalutamide arm in PROSPER [24]. Through an anchored MAIC of HRQoL between the apalutamide and enzalutamide arms of SPARTAN and PROSPER, the present study complemented the findings from the two studies, suggesting that apalutamide may provide additional HRQoL benefits relative to enzalutamide.
The present study has many strengths. The Bayesian MAIC approach used here accounts for differences in measured baseline characteristics in contrast to a previous indirect comparison of apalutamide and enzalutamide conducted by Wallis et al., which did not adjust for baseline characteristics [13]. The Bayesian MAIC approach is different from the Bucher technique used by Wallis et al. and Nieto-Gomez et al. [12, 14], with the latter likely to produce more biased estimates when key baseline characteristics differ (e.g., PSADT) [37]. Furthermore, the probabilistic interpretation of the Bayesian approach enables stating the extent to which a hypothesis is true or false. For instance, in the current study, there was an 89.6% probability of reduced occurrence of SAEs with apalutamide versus enzalutamide in nmCRPC patients. This approach is more relevant for clinical and reimbursement decision-making than the classic frequentist approach, which dichotomizes results to be either significant or non-significant, based on the chosen significance threshold, and does not indicate the probability of the hypothesis being true or false [37]. Moreover, the analyses presented here provide a high level of granularity on AEs and HRQoL, with multiple individual AEs analyzed as well as multiple subscales and composite scores of the FACT-P. Since the value of different aspects of HRQoL and AEs may vary among individual patients (e.g., due to existing comorbidities), the granularity of the analysis may be especially useful in clinical decision-making. As nmCRPC patients are largely asymptomatic, limiting the burden of any treatment while maximizing HRQoL benefit is of clinical relevance when initiating a treatment for these patients, especially since treatment duration might extend over multiple years.
The present study is subject to some limitations. Although potential for biases was substantially reduced after matching, unobserved or unmeasured confounders may impact the relative effect of treatments on the outcomes of interest. Both studies are ongoing, and further analyses will be needed to confirm the potential advantages of apalutamide versus enzalutamide with respect to tolerability and long-term health effects, including HRQoL, over significantly longer follow-up periods. The analyses performed to compare AEs used data from the ITT population because of the unavailability of baseline characteristics for patients in the safety population. However, in both studies, the sizes of the safety and ITT populations differed only slightly, suggesting that any differences in baseline characteristics between the two populations should be minimal. It is important to note that AEs of interest were only analyzed if reported in both studies. For example, rash (apalutamide arm: 23.8%; ADT arm: 5.5%), hypothyroidism (apalutamide arm: 8.1%; ADT arm: 2.0%), and seizures (apalutamide arm: 0.2%; ADT arm: 0) were only assessed in SPARTAN and were therefore not included in the current analysis. More specifically, a post hoc analysis of the SPARTAN study in patients with and without rash was conducted. The median start and stop times of reported rash were evaluated and compared with FACT-P and EuroQol 5 dimension (EQ-5D) scores to get a sense of the impact of rash on HRQoL. No differences were observed between groups. In addition, even though short-term (≤ 4 weeks) administration of corticosteroids was permitted in SPARTAN, the extent of their use in PROSPER was not known. Moreover, AEs and HRQoL were assessed at different time intervals in the two studies, with more frequent assessments in SPARTAN compared with PROSPER. Further, HRQoL changes were only compared at baseline versus end of follow-up. Therefore, it is possible that a selection bias in favor of patients with a good tolerability profile was introduced by the exclusion of patients who discontinued therapy. Finally, follow-up time at which HRQoL was assessed differed slightly between the studies (96 weeks for SPARTAN and 97 weeks for PROSPER).
Conclusion
In this MAIC study, the tolerability and HRQoL in men with nmCRPC who received apalutamide and enzalutamide were indirectly compared using individual patient data from the SPARTAN study [2] and aggregate data from the PROSPER study [1, 25]. This study demonstrates with high probability that apalutamide has more favorable tolerability than enzalutamide, as evidenced by reduced occurrence of most of the individual AEs assessed, including fatigue, hypertension, decreased appetite, fall, and headaches, as well as SAEs. The analyses also revealed a 73.1% probability of a better HRQoL with apalutamide relative to enzalutamide as assessed using the FACT-P total score. Further research is warranted to better understand patients’ experience with these new treatments.
References
Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465–74.
Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408–18.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology, prostate cancer - version 4.2019. Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed 1 Nov 2019.
US Food and Drug Administration. Prescribing information—apalutamide. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210951s000lbl.pdf. Accesssed 18 Feb 2019.
US Food and Drug Administration. Prescribing information—enzalutamide. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203415Orig1s014lbl.pdf. Accesssed 30 Oct 2019.
European Medicine Agency. Summary of product characteristics—Xtandi. https://www.ema.europa.eu/documents/product-information/xtandi-epar-product-information_en.pdf. Accesssed 19 Feb 2019.
European Medicine Agency. Summary of product characteristics—ERLEADA. https://www.ema.europa.eu/en/documents/product-information/erleada-epar-product-information_en.pdf. Accesssed 19 Feb 2019.
American Urological Association. Clinical guidelines: castration-resistant prostate cancer 2013 amended 2018. https://www.auanet.org/guidelines/prostate-cancer-castration-resistant-guideline. Accesssed 6 Mar 2018.
Mottet N, van den Bergh RCN, Briers E et al. European Association of Urology guidelines—prostate cancer 2019. https://uroweb.org/guideline/prostate-cancer/. Accesssed 15 May 2019.
Rozet F, Hennequin C, Beauval JB, et al. Recommandations francaises du Comite de Cancerologie de l’AFU—actualisation 2018–2020: cancer de la prostate French ccAFU guidelines—update 2018–2020: prostate cancer. Prog Urol. 2018;28(12S):S79–130.
Chowdhury S, Oudard S, Hadaschik BA et al. Matching-adjusted indirect comparison of the efficacy of apalutamide and enzalutamide in the treatment of non-metastatic castration-resistant prostate cancer. ISPOR Europe; November 10–14, 2018; Barcelona, Spain.
Nieto-Gomez P, Ubago-Perez R, Cabeza-Barrera J. Efficacy of enzalutamide and apalutamide in the treatment of non-metastasic castration-resistant prostate cancer: indirect comparison. Actas Urol Esp. 2019;43(7):355–63.
Wallis CJD, Thenappan C, Goldberg H, et al. Advanced androgen blockage in nonmetastatic castration-resistant prostate cancer: an indirect comparison of apalutamide and enzalutamide. Eur Urol Oncol. 2018;1(3):238–41.
Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–91.
Ishak KJ, Proskorovsky I, Benedict A. Simulation and matching-based approaches for indirect comparison of treatments. PharmacoEconomics. 2015;33(6):537–49.
Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–7.
Tremblay G, Chandiwana D, Dolph M, Hearnden J, Forsythe A, Monaco M. Matching-adjusted indirect treatment comparison of ribociclib and palbociclib in HR+, HER2− advanced breast cancer. Cancer Manag Res. 2018;10:1319–27.
Badia X, Herdman M. The importance of health-related quality-of-life data in determining the value of drug therapy. Clin Ther. 2001;23(1):168–75.
Torvinen S, Farkkila N, Sintonen H, Saarto T, Roine RP, Taari K. Health-related quality of life in prostate cancer. Acta Oncol. 2013;52(6):1094–101.
Efficace F, Rees J, Fayers P, et al. Overcoming barriers to the implementation of patient-reported outcomes in cancer clinical trials: the PROMOTION Registry. Health Qual Life Outcomes. 2014;12:86.
Nipp RD, El-Jawahri A, Moran SM, et al. The relationship between physical and psychological symptoms and health care utilization in hospitalized patients with advanced cancer. Cancer. 2017;123(23):4720–7.
Body JJ, Casimiro S, Costa L. Targeting bone metastases in prostate cancer: improving clinical outcome. Nat Rev Urol. 2015;12(6):340–56.
Saad F, Cella D, Basch E, et al. Effect of apalutamide on health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: an analysis of the SPARTAN randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(10):1404–16.
Tombal B, Saad F, Penson D, et al. Patient-reported outcomes following enzalutamide or placebo in men with non-metastatic, castration-resistant prostate cancer (PROSPER): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(4):556–69.
Saad F, Penson D, Attard G et al. MP52-16: impact of enzalutamide on pain and health-related quality of life in men with non-metastatic castration-resistant prostate cancer: PROSPER study results. American Urological Association Annual Meeting; May 18–21, 2018; San Francisco, CA, USA.
Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50(6):920–8.
Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–9.
Sellers L, Savas AN, Davda R, Ricketts K, Payne H. Patient-reported outcome measures in metastatic prostate cancer. Trends Urol Men’s Health. 2016;7(1):28–32.
Yount S, Cella D, Banik D, Ashraf T, Shevrin D. Brief assessment of priority symptoms in hormone refractory prostate cancer: the FACT Advanced Prostate Symptom Index (FAPSI). Health Qual Life Outcomes. 2003;1:69.
Cella D, Nichol MB, Eton D, Nelson JB, Mulani P. Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy–Prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health. 2009;12(1):124–9.
Signorovitch JE, Wu EQ, Yu AP, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. PharmacoEconomics. 2010;28(10):935–45.
Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33(5):607–17.
National Institute for Health and Care Excellence. Technology appraisal guidance 2017. https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-technology-appraisal-guidance. Accesssed 6 Mar 2018.
Philippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submissions to NICE: NICE; 2016. http://nicedsu.org.uk/wp-content/uploads/2017/05/Population-adjustment-TSD-FINAL.pdf. Accesssed 30 Oct 2018.
Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Eng J Med. 2014;371(5):424–33.
Gartrell BA, Saad F. Managing bone metastases and reducing skeletal related events in prostate cancer. Nat Rev Clin Oncol. 2014;11(6):335–45.
Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14(4):417–28.
Acknowledgements
Funding
This study was funded by Janssen Scientific Affairs, LLC. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. The journal’s Rapid Service Fee and Open Access publication were purchased using funding provided by the study sponsor.
Medical Writing Assistance
Medical writing assistance was provided by Samuel Rochette and Sara Kaffashian, who are employees of Analysis Group, Inc., which provided paid consulting services to Janssen Scientific Affairs for the conduct of the present study.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentation
Part of the material in this manuscript was presented at the ISPOR Europe 2018 Conference, November 10–14, 2018, Barcelona, Spain.
Disclosures
Dominic Pilon is an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC., which funded the development and conduct of this study and manuscript. Patrick Lefebvre is an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC., which funded the development and conduct of this study and manuscript. Kelly McQuarrie is an employee of Janssen Scientific Affairs, LLC., and may own stocks or stock options. Jinan Liu is an employee of Janssen Scientific Affairs, LLC., and may own stocks or stock options. Lindsay Dearden is an employee of Janssen Global Services and may own stocks or stock options. Jan Sermon is an employee of Janssen EMEA and may own stocks or stock options. Suzy Van Sanden is an employee of Janssen EMEA and may own stocks or stock options. Boris A. Hadaschik reports advisory roles for Bayer, Lightpoint Medical, Inc., Janssen R&D, Bristol-Myers-Squibb and Astellas; research funding from Profound Medical, German Cancer Aid, German Research Foundation, Janssen R&D, Bristol-Myers-Squibb and Astellas; and travel from AstraZeneca, Janssen R&D and Astellas.
Compliance With Ethics Guidelines
The SPARTAN study is a Janssen-sponsored study, and all appropriate ethics approvals were granted. Data from the PROSPER study were obtained from publicly available sources. Review boards at participating institutions approved the SPARTAN and PROSPER studies, and they were conducted in accordance with the current International Conference on Harmonisation guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.10283561.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chowdhury, S., Oudard, S., Uemura, H. et al. Matching-Adjusted Indirect Comparison of Health-Related Quality of Life and Adverse Events of Apalutamide Versus Enzalutamide in Non-Metastatic Castration-Resistant Prostate Cancer. Adv Ther 37, 512–526 (2020). https://doi.org/10.1007/s12325-019-01157-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-01157-4