Abstract
Introduction
The interaction between anticoagulants and platelet function is complex. Previous publications showed mixed results regarding the role of heparins in platelet aggregation. On the other hand, the direct thrombin inhibitor (DTI) dabigatran might enhance the risk of myocardial infarction in patients with atrial fibrillation, which could be related to increased platelet aggregability.
Methods
This was a prospective, interventional study of patients with chronic coronary artery disease (CAD) taking low-dose aspirin. The objective of the current study was to compare the effects of dabigatran versus enoxaparin on platelet aggregability. Subjects initially were on orally administered dabigatran for 5 days followed by subcutaneously administered enoxaparin after a 30-day washout period. Platelet function was assessed at baseline and after each intervention by multiple electrode aggregometry (MEA-ASPI) (primary endpoint), serum thromboxane B2 (TXB2), VerifyNow Aspirin™, and coagulation tests (secondary endpoints).
Results
Compared to baseline MEA-ASPI values, dabigatran increased platelet aggregation while enoxaparin decreased platelet aggregation (+ 5 U ± 24.1 vs − 6 U ± 22.2, respectively, p = 0.012). The TXB2 assay showed the same pattern (+ 2 pg/ml for dabigatran vs − 13 pg/ml for enoxaparin, p = 0.011). None of the additional tests showed significant differences between the groups. Individually, compared to baseline TXB2 results, enoxaparin significantly decreased platelet activation [33 (16.5–95) pg/mL vs 20 (10–52) pg/mL, respectively, p = 0.026], but no significant differences were observed with dabigatran.
Conclusions
DTI and anti-Xa drugs exert opposite effects on platelet function. A significant decrease in platelet activation through COX1 (also known as prostaglandin G/H synthase 1) was observed with enoxaparin, but no significant differences in platelet function were observed with dabigatran.
Trial Registration
ClinicalTrials.gov identifier, NCT02389582.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The interaction between different anticoagulants and platelet function is controversial. |
Direct thrombin inhibitors can paradoxically enhance the risk of cardiovascular events in patients with atrial fibrillation. |
Different targeting anticoagulants might have opposite effects on platelet aggregation. |
What was learned from the study? |
There is no influence of dabigatran on pharmacodynamics effects of aspirin on patients with chronic coronary artery disease. |
Anti-Xa drugs can reduce platelet aggregation. |
Introduction
The interaction between anticoagulants and platelet activation is complex. The apparent paradoxical association of anticoagulants use with coronary and/or mechanical heart prothesis thromboses has been previously reported [1,2,3,4].
Although the role of anticoagulants in the secondary prevention of recurrent ischemic events after acute coronary syndrome (ACS) and during percutaneous coronary intervention (PCI) in stable coronary artery disease (CAD) is well established [5,6,7,8], the possible increase in ischemic coronary events with anticoagulants in different clinical settings is a matter of concern [2, 6, 9, 10]. In this context, enoxaparin, a low molecular weight heparin (LMWH) with predominant anti-Xa mechanism, was shown to be safe in other studies, without pro-aggregatory effects [4, 5, 11, 12]. In contrast, previous data on different direct thrombin inhibitors (DTI) suggest elevated platelet-mediated risk of thrombosis. Laboratory data from the phase II PETRO trial have shown that dabigatran increases urinary thromboxane metabolite excretion, indicating a platelet‐activating effect [13]. Additionally, data from the RE-LY trial, which tested dabigatran versus warfarin in patients with atrial fibrillation (AF), suggested that dabigatran may increase the risk of myocardial infarction (MI) (in comparison with warfarin, HR = 1.35, p = 0.07 for the 110 mg dose and HR 1.38, p = 0.048 for the 150 mg dose) [1]. Also, bivalirudin, a parenterally administered DTI, increased the risk of acute stent thrombosis within 24 h in patients with ST-segment elevation MI when compared with heparin plus glycoprotein IIb/IIIa inhibitors [6]. On the other hand, the efficacy results from the randomized placebo-controlled study of dabigatran in patients with ACS on dual antiplatelet therapy (DAPT) were inconclusive. Although there were numerically more cases of MI in the dabigatran arm in the RE-DEEM study, this finding was not statistically significant [14].
Thus, we hypothesized that DTI (dabigatran) and anti-Xa inhibitor (enoxaparin) might exert antagonistic effects on platelet aggregation in a population of patients with stable CAD.
Methods
Study Design, Setting, and Population
This was a prospective, open-label, interventional crossover study conducted at the Heart Institute (InCor/HCFMUSP), University of São Paulo Medical School, in patients with chronic CAD. Patients were retrospectively and randomly selected from the Acute Coronary Disease Unit databank. The population consisted of both genders, of least 18 years of age with CAD on aspirin 100 mg/day. CAD was defined as one or more of the following: previous MI, coronary angioplasty, coronary artery bypass graft (CABG) surgery, or coronary angiography showing obstruction of at least 50% in one major epicardial vessel. The main exclusion criteria were as follows: use for the last 7 days of an orally administered anticoagulant or any other antiplatelet drug aside from ASA, any active bleeding, pregnancy or the lack of a contraceptive method in women of childbearing age, hemoglobin < 10 g/dL, hematocrit < 30% or > 50%, platelet count < 100,000/mm3 or > 500,000/mm3, moderate renal insufficiency (creatinine clearance < 50 ml/min), PCI during the last 30 days before randomization (or PCI during the last year when drug-eluting stents were used), CABG during the last 90 days, ACS during the last 60 days, active malignant neoplasm, active peptic ulcer disease during the last 60 days or upper gastrointestinal bleeding at any time and AF.
The study was approved by the Institutional Review Board of the Ethics Committee (Comissão de Ética para Análise de Projetos de Pesquisa do HCFMUSP) and was conducted in accordance with the declaration of Helsinki of 1964 and its later amendments. All participants provided written informed consent for both their participation in this study and for its publication. The study was registered at ClinicalTrials.gov (NCT02389582).
Study Procedures
Patients were submitted to medical interview and physical examination to assess the eligibility criteria. If selected, blood samples were collected for the evaluation of platelet function and for general laboratory tests at baseline. All patients were assigned to dabigatran (150 mg twice daily) for 5 days, followed by a washout period of 30 days. Next, patients were submitted to enoxaparin (1 mg/kg twice daily) for an additional 5 days. Enoxaparin’s choice as the anti-Xa drug was due to its safety and the lack of orallly administered anti-Xa adequate doses for the CAD scenario in Brazil. Platelet function tests were performed at baseline and after both treatments (dabigatran and enoxaparin) (Fig. 1) [4, 5].
Multiplate electrode aggregometry (MEA) tests (Dynabyte Medical, Munich, Germany) were performed for whole blood platelet aggregometry. Platelet aggregation was triggered with arachidonic acid (MEA-ASPI test) and thrombin receptor-activating peptide 6 (MEA-TRAP test). Reagent delivery was performed using an automatic pipette, and aggregation was recorded for 6 min. The increase in impedance because of the attachment of platelets to the electrodes was detected for each sensor unit separately. Aggregation measured by MEA was quantified as the area under the aggregation curve [AUC, (AU min)] and expressed in units (U), where 10 AU min corresponds to 1 U [15].
VerifyNow (VN) Aspirin™ blood samples were collected in anticoagulant-coated tubes with 3.2% sodium citrate (Vacutainer™), which were placed inside a cartridge for the assay. Reading occurred in the automated system after 5 min, and the results are described in aspirin reactivity units (ARUs).
Serum thromboxane B2 (TXB2) was measured to assess COX1 (also known as prostaglandin G/H synthase 1) inhibition by aspirin. The blood was collected in tubes without anticoagulant and incubated for 1 h, allowing the whole blood to clot and generate thrombin. The serum was then stored at − 80 °C and measured in duplicate by ELISA (Cayman Chemical, Michigan, EUA) according to the manufacturer’s instructions.
In order to test for an adequate anticoagulation response, coagulation assays and kinetics regarding clot formation (coagulation time and clot formation time) and clot strength (maximum clot firmness) were studied with free oscillation rheometry (FOR), assessed with the ReoRox G2® rheometer (Medirox AB, Nyköping, Sweden). FOR utilizes an oscillating movement to monitor coagulation. The sample is added to a reaction chamber, which consists of a gold-coated sample cup with a gold-coated cylinder (bob) suspended in the blood sample. FOR uses a torsion wire system to set the sample into oscillation. A magnet pulls back the measuring head connected to the torsion wire. On release, the torsion wire will set the cup into free oscillation and its movement is recorded by an optical detector. The changes of damping and frequency of the oscillation correlate to viscosity and elasticity, respectively, which are recorded as a viscosity curve and an elasticity curve.
Study Objectives
The primary objective of the study was to compare dabigatran and enoxaparin regarding platelet aggregation using the MEA-ASPI test. The secondary objectives included the same evaluation with additional platelet function tests, namely, MEA-TRAP, TXB2, and VN Aspirin™ assays and coagulation tests. Additionally, the individual effects of enoxaparin and dabigatran on platelet aggregation were assessed by the MEA-ASPI test and compared to baseline values.
Statistical Analysis
The sample size was calculated assuming a difference between the drugs of 34% in the value of platelet aggregation, according to a previous publication [4]. A statistical significance level of 95% was estimated, as well as a power of 80% and a loss to follow-up of 10%, for a sample size of 28 patients.
Categorical variables are described as absolute numbers or percentages and were compared using the chi-square test or Fisher’s exact test, as appropriate. Continuous variables are described with their respective mean and standard deviations (assuming a Gaussian distribution) or their median values and 25th and 75th percentiles. The Kolmogorov–Smirnov test was used to evaluate normality. The paired Student’s t test (normal distribution) or Wilcoxon (non-Gaussian distribution) test was used to compare platelet function between the groups.
The statistical analyses were performed using SPSS 17.0 (Microsoft, Chicago, USA), and the level of significance was defined as p < 0.05.
Results
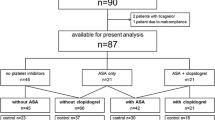
From the Acute Coronary Disease Unit databank, we retrospectively evaluated 86 patients which, accordingly to the available variables, were potential candidates for the study. As Fig. 2 shows, 31 patients were initially selected; two of them met the exclusion criteria after the baseline visit because of platelet count (< 100,000/mm3) and moderate renal insufficiency (creatinine clearance < 50 ml/min).
The baseline patient characteristics showed a mean age of 63 ± 8 years, and 69% were male. Regarding their medical history, 79.3% had arterial hypertension, and 48% were diabetic, with a median HbA1c of 6.2%. All patients had previous MI, the majority non-STEMI, and 75% had previously undergone a PCI. All patients were on statins, and 89.7% were taking beta-blockers (Table 1).
Primary Objective
The DTI dabigatran increased platelet aggregability by 5 U ± 24.1 when compared to the anti-Xa enoxaparin, which decreased platelet aggregability by 6 U ± 22.2 (p = 0.012, 95% CI 2.4–18.1), with respect to the baseline values, as measured by MEA-ASPI test (Fig. 3).
Secondary Objectives
Likewise the MEA-ASPI results in Fig. 3 show a significant difference between both therapy groups regarding serum TXB2 levels, which increased with dabigatran and decreased with enoxaparin (+ 2 pg/ml for dabigatran vs − 13 pg/ml for enoxaparin, p = 0.011).
Figure 4 shows the results of the comparison between baseline and after treatment for each drug studied, considering different tests: for MEA-ASPI, there was no statistically significant difference for dabigatran or enoxaparin; on the other hand, TXB2 levels were significantly reduced after enoxaparin treatment compared to baseline [20 (10–52) pg/ml after treatment versus 33 (16.5–95) pg/ml at baseline, p = 0.026], but no significant differences were observed with dabigatran [33 (16.5–95) pg/ml versus 35 (19.5–90), p = 0.602]; for the other tests, Table 2 shows that MEA-TRAP and VN Aspirin™ presented no differences in platelet aggregation between the groups.
Regarding the blood coagulation tests (Table 3), the comparison between dabigatran and enoxaparin showed significant differences for all the analyzed parameters, with a more pronounced effect of dabigatran on mean activated partial thromboplastin time, international normalized ratio for prothrombin time, coagulation time, and clot formation time (all p values < 0.0001); and a less pronounced dabigatran effect on the maximum clot firmness (p = 0.027).
Discussion
In this study, an increase in platelet aggregation was observed after dabigatran treatment compared to enoxaparin treatment in patients with CAD on aspirin, as assessed by the MEA-ASPI test.
The effects of dabigatran on platelet function and clinical outcomes have been a matter of concern since several trials suggested increased MI and/or ischemia with the use of the drug [1, 9, 10, 16, 17]. Additionally, evidence also suggests that small DTI, such as dabigatran and bivalirudin, could increase the risk of thrombosis [18, 19].
Nevertheless, it is important to emphasize that no significant effect of dabigatran on platelet aggregation was found when compared to baseline, as assessed by different methods. This finding could be due to the concomitant use of aspirin, as shown in other trials of antiplatelet therapy and the use of new orallly administered anticoagulants, with no significant effect on platelet function [20, 21].
On the other hand, recent data have shown that dabigatran increases platelet reactivity by increasing the thrombin receptor density on platelets, as measured by platelet thrombin receptor expression [22]. These findings were not confirmed in the present study, since no significant differences were noticed in platelet aggregation after treatment with dabigatran or enoxaparin when the TRAP receptor level (MEA-TRAP test) was assessed. This last result is also in accordance with the clinical setting, where different observational trials have suggested the safety of dabigatran in patients with AF [23, 24]. In addition, in patients with nonvalvular AF undergoing PCI, the RE-DUAL PCI trial evaluated dual antithrombotic therapy with dabigatran and a P2Y12 inhibitor versus triple therapy with warfarin plus DAPT and found no differences between the groups regarding thromboembolic events. Although the absolute number of MIs was higher for both doses of dabigatran, with 44 patients (4.5%) in the 110-mg dual-therapy group compared with 29 (3%) in the triple-therapy group and 26 patients (3.4%) in the 150-mg dual-therapy group compared with 22 (2.9%) in the corresponding triple-therapy group, the observed differences between groups did not reach statistical significance [25].
Finally, this study’s data on enoxaparin shows a reduction in platelet activation by MEA-ASPI and TXB2 when compared with baseline, with similar results for dabigatran, as tested with the TXB2 assay. This result may be due to different mechanisms of action, such as decreasing the ability of platelets to bind fibrinogen (glycoprotein IIb/IIIa activation) and express P-selectin in response to adenosine diphosphate [26, 27]. In contrast, this study’s results could not be reproduced in a clinical setting as, compared to dabigatran, enoxaparin did not reduce myocardial ischemic events in different trials reported in a metanalysis by Douxfils et al. [9].
Regarding coagulation tests, the present results are in compliance with the literature, as dabigatran, but not enoxaparin, prolonged the aPTT, thus increasing the aPTT ratio. It is important to notice that aPTT may provide a qualitative assessment of the dabigatran level and activity by a curvilinear relation and is a considerable tool for guiding dabigatran reversal [28, 29].
Concerning the coagulation kinetics, this study’s findings are consistent with previous data that show a significant linear correlation between the clotting time and dabigatran concentration, which might be useful for anticoagulation monitoring and emergency management [30].
Study Limitations
This study’s results should be interpreted considering the specific characteristics of the population composed of patients with chronic CAD on aspirin. Therefore, the results should not be extrapolated to patients with DAPT indications or populations with AF not taking aspirin. In addition, this was a mechanistic study evaluating a surrogate outcome. Thus, it is not possible to make definitive conclusions regarding the clinical impact of the findings.
Conclusion
No significant differences in platelet aggregation were observed with dabigatran compared to baseline in patients with CAD on aspirin, reinforcing its safety in terms of final platelet activity. However, when tested against the anti-Xa effect of enoxaparin, dabigatran increased platelet activation and aggregation promoted by thromboxane. Furthermore, enoxaparin showed a significant decrease in platelet activation by COX1 when analyzed by TXB2.
References
Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51.
Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–14.
Fiessinger JN, Huisman MV, Davidson B, et al. Ximelagatran vs low-molecular-weight heparin and warfarin for the treatment of deep vein thrombosis: a randomized trial. JAMA. 2005;293:681–9.
Xiao Z, Theróux P. Platelet activation with unfractionated heparin at therapeutic concentrations and comparison with low-molecular-weight heparin and with a direct thrombin inhibitor. Circulation. 1998;97:251–6.
Ferguson J, Califf R, Atman E, et al. Enoxaparin vs unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy. JAMA. 2004;292:45–54.
Stone GW, Witzenbichler B, Guagliumi G, et al. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358:2218–30.
Gargiulo G, Carrara G, Frigoli E, et al. Bivalirudin or heparin in patients undergoing invasive management of acute coronary syndromes. J Am Coll Cardiol. 2018;71:1231–42.
Valgimigli M, Frigoli E, Leonardi S, et al. Bivalirudin or unfractionated heparin in acute coronary syndromes. N Engl J Med. 2015;373:997–1009.
Douxfils J, Buckinx F, Mullier F, et al. Dabigatran etexilate and risk of myocardial infarction, other cardiovascular events, major bleeding, and all-cause mortality: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2014;3:e000515.
Hohnloser SH, Oldgren J, Yang S, et al. Myocardial ischemic events in patients with atrial fibrillation treated with dabigatran or warfarin in the RE-LY (Randomized evaluation of long-term anticoagulation therapy) trial. Circulation. 2012;125:669–76.
Antman EM, Morrow DA, McCabe CH, et al. Enoxaparin versus unfractionated heparin with fibrinolysis for ST-elevation myocardial infarction. N Engl J Med. 2006;354:1477–88.
Montalescot G, Bal-dit-Sollier C, Chibedi D, et al. Comparison of effects on markers of blood cell activation of enoxaparin, dalteparin, and unfractionated heparin in patients with unstable angina pectoris or non-ST-segment elevation acute myocardial infarction (the ARMADA study). Am J Cardiol. 2003;91:925–30.
Ezekowitz MD, Reilly PA, Nehmiz G, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study). Am J Cardiol. 2007;100:1419–26.
Oldgren J, Budaj A, Granger CB, et al. Dabigatran vs. placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized, double-blind, phase II trial. Eur Heart J. 2011;32:2781–9.
Würtz M, Hvas AM, Christensen KH, Rubak P, Kristensen SD, Grove EL. Rapid evaluation of platelet function using the Multiplate® Analyzer. Platelets. 2014;25:628–33.
Connolly S, Ezekowitz M, Yusuf S, Reilly P, Wallentin L. Newly identified events in the RE-LY trial. N Engl J Med. 2010;363:1875–6.
Hohnloser SH, Lip GYH. Dabigatran and myocardial infarction. Chest. 2015;147:e70–1.
Franchi F, Rollini F, Rae Cho J, et al. Effects of dabigatran on the cellular and protein phase of coagulation in patients with coronary artery disease on dual antiplatelet therapy with aspirin and clopidogrel. Results from a prospective, randomised, double-blind, placebo-controlled study. Thromb Haemost. 2016;115:622–31.
Laine M, Frere C, Cuisset T, et al. Potential mechanism of acute stent thrombosis with bivalirudin following percutaneous coronary intervention in acute coronary syndromes. Int J Cardiol. 2018;220:496–500.
Olivier CB, Weik P, Meyer M, et al. Dabigatran and rivaroxaban do not affect AA- and ADP-induced platelet aggregation in patients receiving concomitant platelet inhibitors. J Thromb Thrombolysis. 2016;42:161–6.
Dans AL, Connolly SJ, Wallentin L, et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the randomized evaluation of long-term anticoagulation therapy (RE-LY) trial. Circulation. 2013;127:634–40.
Achilles A, Mohring A, Dannenberg L, et al. Dabigatran enhances platelet reactivity and platelet thrombin receptor expression in patients with atrial fibrillation. J Thromb Haemost. 2017;15:473–6.
Graham DJ, Reichman ME, Wernecke M, et al. Cardiovascular, bleeding, and mortality risks in elderly medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2014;131:157–64.
Larsen TB, Rasmussen L, Skjøth F, et al. Efficacy and safety of dabigatran etexilate and warfarin in “real-world” patients with atrial fibrillation a prospective nationwide cohort study. J Am Coll Cardiol. 2013;61:2264–73.
Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513–24.
Aggarwal A, Whitaker D, Rimmer J, et al. Attenuation of platelet reactivity by enoxaparin compared with unfractionated heparin in patients undergoing haemodialysis. Nephrol Dial Transpl. 2004;19:1559–63.
Aggarwal A, Sobel B, Schneider D. Decreased platelet reactivity in blood anticoagulated with bivalirudin or enoxaparin compared with unfractionated heparin: implications for coronary intervention. J Thromb Thrombolysis. 2002;13:161–5.
Solbeck S, Meyer MAS, Johansson PI, et al. Monitoring of dabigatran anticoagulation and its reversal in vitro by thrombelastography. Int J Cardiol. 2014;176:794–9.
Pollack CV, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal—full cohort analysis. N Engl J Med. 2017;377:431–41.
Taune V, Wallén H, Ågren A, et al. Whole blood coagulation assays ROTEM and T-TAS to monitor dabigatran treatment. Thromb Res. 2017;153:76–82.
Acknowledgements
We thank all participants of the study.
Funding
This study was supported by grants from São Paulo Research Foundation (FAPESP), São Paulo, Brazil. The funding source had no role in study design, decision for publication or data analyses. No Rapid Service Fee was received by the journal for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Authorship Contributions
José C. Nicolau: conception and design, interpretation of data, drafting the study manuscript. Flavia B. B. Arantes: conception and design, acquisition and interpretation of data, drafting the study manuscript. All the other authors participated of interpretation of data and critically revising the manuscript.
Prior Presentations
This original research was presented as an abstract poster at the American College Cardiology Scientific Sessions in 2017.
Disclosures
Flavia B.B. Arantes: Research Grant: Novo Nordisk, DalCor, AstraZeneca, Novartis. Talia F. Dalcoquio: Travel grant: Bayer; Research Grant: AstraZeneca, DalCor. Remo H.M. Furtado: Honoraria: AstraZeneca; Research Grant: AstraZeneca, DalCor, Boehringer, Pfizer, Bayer, Sanofi. Luciano M. Baracioli: Research Grant: AstraZeneca, DalCor. José C. Nicolau: Research Grant from São Paulo Research Foundation and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES); personal fees from AMGEN, grants from AstraZeneca, grants and personal fees from Bayer, grants from Bristol-Meyers-Squibb, grants from CLS Behring, personal fees from Daiichi-Sankyo, grants from Dalcor, grants from Janssen, grants and personal fees from Novartis, grants from NovoNordisk, grants and personal fees from Sanofi, personal fees from Servier, grants from Vifor. Fernando R. Menezes, Andre Franci, Carlos J.D.G. Barbosa, Carlos A.K. Nakashima, Quintiliano S.S. Nomelini, José A.F. Ramires, Roberto Kalil Filho have nothing to disclose.
Compliance with Ethics Guidelines
The study was approved by the Institutional Review Board of the Ethics Committee (Comissão de Ética para Análise de Projetos de Pesquisa do HCFMUSP) and was conducted in accordance with the declaration of Helsinki of 1964 and its later amendments. All participants provided written informed consent for both their participation in this study and for its publication. The study was registered at ClinicalTrials.gov (NCT02389582).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.10001921.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Arantes, F.B.B., Menezes, F.R., Franci, A. et al. Influence of Direct Thrombin Inhibitor and Low Molecular Weight Heparin on Platelet Function in Patients with Coronary Artery Disease: A Prospective Interventional Trial. Adv Ther 37, 420–430 (2020). https://doi.org/10.1007/s12325-019-01153-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-01153-8