Abstract
Introduction
Tiotropium, a long-acting muscarinic antagonist, is approved for maintenance treatment of asthma in patients at least 6 years of age in the USA. We systematically reviewed published evidence on the efficacy and safety of 2.5 µg tiotropium Respimat® add-on therapy to inhaled corticosteroid (ICS) with or without additional controller medication(s) in children, adolescents, and adults with asthma.
Methods
We searched PubMed from inception until October 3, 2018, for phase 2 and 3 randomized controlled trials (RCTs) evaluating the effects of 2.5 µg tiotropium Respimat® on lung function parameters in patients with asthma. We extracted adjusted mean differences for lung function data and adverse events (AEs) from relevant articles.
Results
Overall, 11 RCTs (three phase 2 and eight phase 3 studies) including 3244 patients (2.5 µg tiotropium Respimat®, n = 1642; placebo, n = 1602) met the predefined inclusion criteria. Once-daily 2.5 µg tiotropium Respimat® improved lung function parameters, including peak and trough forced expiratory volume in 1 s and peak and trough forced vital capacity, versus placebo. Overall, the safety profile of 2.5 µg tiotropium Respimat® was comparable to that of placebo, with the most commonly reported AEs being asthma worsening, reduction in peak expiratory rate, nasopharyngitis, and respiratory tract infections.
Conclusion
On the basis of the results of phase 2 and 3 studies, 2.5 µg tiotropium Respimat® as add-on to ICS therapy was safe and associated with consistent improvements in lung function in patients with asthma of varying severities across different age groups.
Funding
Development of the manuscript was funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asthma, one of the most common chronic respiratory conditions, poses a substantial human and socioeconomic burden. In the USA alone, approximately 20.4 million adults and 6.1 million children—or 8.3% of the population—were reported to have asthma in 2016 [1]. A recent study based on the Medical Expenditure Panel Survey showed that the economic burden of asthma in the USA was estimated to exceed $80 billion in 2013 [2]. Typical signs and symptoms of asthma, such as wheezing, dyspnea, chest tightness, and cough, which reflect episodes of reversible airflow obstruction, may remit spontaneously or with treatment. However, many patients experience progressive airway remodeling, leading to an incompletely reversible, or fixed, airflow obstruction [3]. In certain situations, such as exposure to allergens and respiratory infections, patients can experience a flare-up of asthma signs and symptoms or asthma exacerbations [4].
Asthma is generally classified as allergic or nonallergic and ranges in severity from mild to severe [5], with severe asthma being associated with higher morbidity and mortality [6]. Susceptibility to and/or development of asthma involves a complex interplay of individual characteristics and environmental factors. Variability in immunologic responses (endotypes) results in different pathophysiological characteristics (phenotypes), in turn contributing to asthma heterogeneity [3]. Moreover, asthma affects patients of all ages and can develop at any time from childhood (early onset) to late in adulthood (late onset), making the diagnosis and management of asthma sometimes challenging, particularly in children [7].
In general, diagnosis of asthma is based on patient history (such as characteristic signs and symptoms) and clinical evidence of variable expiratory airflow limitation [8]. According to the Global Initiative for Asthma (GINA) 2018 report, an asthma diagnosis should be confirmed using objective assessments such as spirometry, which reveals variable airway obstruction that is at least partially reversible [8]. Most national and international guidelines on asthma management, including GINA, advocate regular use of spirometry in the diagnosis and subsequent management of asthma. Primary care providers (PCPs) often prescribe reliever medications “as needed” on the basis of history and physical examination [9]. Although treatment step-up, as recommended by GINA, is based on the assessment of symptom control, spirometry could help PCPs identify patients with poor perceptions of their symptoms and make data-driven therapeutic decisions for stepping up treatment in symptomatic patients [10].
Asthma pharmacotherapies can be classified as reliever (rescue) and controller (maintenance) medications [8]. Reliever medications provide relief from acute respiratory symptoms during asthma attacks (e.g., short-acting β2-agonists [SABAs], short-acting muscarinic antagonists [SAMAs], and long-acting β2-agonist [LABA]{formoterol}/inhaled corticosteroid [ICS] combinations) [8]. Controller medications provide long-term symptom control, and reduce airway inflammation and the risk of lung function decline and future exacerbations. Such medications include ICSs, SABAs (in combination with an ICS), LABAs (in combination with an ICS), and add-on therapies such as long-acting muscarinic antagonists (LAMAs), leukotriene receptor antagonists (LTRAs), and immunomodulators/biologics (anti-immunoglobulin E [IgE] and anti-interleukin-5/interleukin-5 receptor and anti-interleukin-4 receptor therapies) [8]. However, despite the availability of a wide range of treatment options, approximately 12.4 million (46.9%) patients in the USA—9.1 million (44.9%) adults and 3.3 million (53.7%) children across all asthma severities—continue to experience exacerbations as reported by the 2016 National Health Interview Survey [1]. This finding highlights the need for effective add-on treatments that can improve lung function across the spectrum of asthma patients. Although LABA added to low-dose ICS is more effective in attaining asthma control than increasing the dose of ICS [11], LABA/ICS can achieve well-controlled asthma in only approximately 70% of patients [12].
Tiotropium Respimat® (Spiriva® Respimat® inhalation spray; Boehringer Ingelheim, Ridgefield, CT, USA) is a LAMA approved for long-term, once-daily, maintenance treatment of asthma in patients aged at least 6 years (2.5 µg [two puffs of 1.25 µg once-daily] in the USA and 5 µg [two puffs of 2.5 µg once-daily] in the European Union [EU] and other countries) [13, 14]. Approvals were based on the results of several phase 3 trials of tiotropium—delivered via HandiHaler®, a dry powder inhaler, or via Respimat®, a slow-mist inhaler (in phase 3 trials and submitted globally to regulatory authorities)—as add-on treatment to ICS with or without other controller medications in patients with uncontrolled or symptomatic asthma [15,16,17,18,19,20,21,22]. Results from these studies demonstrated that add-on treatment with tiotropium Respimat® improved lung function and reduced the risk of severe exacerbations and asthma worsening [15, 20, 22, 23]. Moreover, the efficacy of tiotropium was demonstrated across all age groups (children, adolescents, and adults) and asthma severities (mild, moderate, and severe) [15, 16, 18,19,20,21,22, 24].
Considering that tiotropium HandiHaler® for asthma has been discontinued in the USA, we conducted a systematic review to investigate the efficacy and safety of once-daily 2.5 µg tiotropium Respimat®, the US Food and Drug Administration (FDA)-approved dosage, across all age groups and asthma severities.
Methods
Search Strategy
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [25]. We searched PubMed from inception until October 3, 2018, using the following search terms: asthma*[title] AND tiotropium*[title] AND (study OR trial) NOT review. Literature search results were limited to articles published in English. Reference lists of articles that met the inclusion and exclusion criteria, as well as articles from the authors’ personal files, were reviewed to identify any other relevant citations. The protocol was not prospectively registered on any registry.
Trial Selection
Articles captured during the PubMed search were imported into an EndNote library. Titles and abstracts of all articles were screened by Saurabh Gagangras (S.G.) and Maribeth Bogush (M.B.), and independently verified by Lyndon Mansfield (L.M.), Sy Duong-Quy (S.D.), and Timothy Craig (T.C.).
Articles were included if they met the following criteria: (i) prospective phase 2 or 3 randomized controlled trial (RCT), (ii) delivery of tiotropium via Respimat®, (iii) evaluation of the 2.5 µg tiotropium dose, and (iv) efficacy endpoints related to lung function.
Articles were excluded if they met the following criteria: (i) pooled datasets from multiple trials or in vitro/preclinical evaluations, (ii) narrative reviews, systematic reviews ± meta-analyses, case studies, opinion editorials, and errata (unless pertaining to a relevant study), and (iii) trials with a focus on adherence, asthma–chronic obstructive pulmonary disease overlap, clinical characteristics, comorbidities, epidemiology, health care costs, hospitalizations, monitoring, and quality of life.
After application of the inclusion and exclusion criteria, full texts of the remaining articles (and corresponding reference lists) were reviewed by S.G. and M.B. to identify articles for analysis. The process of categorization of articles meeting the inclusion or exclusion criteria was reviewed by L.M., S.D., and T.C., who also scanned personal files for relevant articles. Any disagreements were resolved by consensus-based discussions. Adjusted mean differences for lung function data [peak and trough forced expiratory volume in 1 s (FEV1), peak and trough forced vital capacity (FVC), morning/evening/peak/trough peak expiratory flow (PEF)] and patient-reported outcomes [PROs; as assessed by the Asthma Control Questionnaire (ACQ)-7 or the Interviewer-Administered Version of the ACQ (ACQ-IA)], and adverse events (AEs) from relevant articles were extracted by S.G. and M.B., and independently reviewed by L.M., S.D., and T.C.
Quality Assessment
The Cochrane risk of bias tool was used to assess the risk of bias in estimating the outcomes from each trial [26]. Each trial was assessed for the following: (i) random sequence generation, (ii) allocation concealment, (iii) blinding of participants and personnel, (iv) blinding of outcome assessment, (v) incomplete outcome data, (vi) selective reporting, and (vii) other biases. Each domain was graded as low, high, or unclear for the potential risk of bias. In cases where data or information was missing from the publication, we contacted the corresponding authors by e-mail to request the full original data.
Statistical Analysis
Relevant data from the selected publications were extracted and forest plots for key endpoints (peak and trough FEV1, peak and trough FVC, morning/evening/peak/trough PEF, and ACQ/ACQ-IA scores) from the included trials were constructed to represent the data graphically.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
Trial Selection
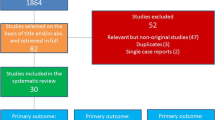
The PubMed search yielded 54 relevant articles, 11 of which were included in the analysis (Fig. 1). After review of the titles and abstracts of all articles, 44 were excluded from the analysis (the output of the search string, along with the reason(s) for inclusion or exclusion for each study are provided in a supplementary worksheet); the majority (68.2%; n = 30) of articles were excluded as they did not meet the inclusion criteria. One article was added following review of the resultant 10 full-text articles, including corresponding reference lists and authors’ personal files.
Trial and Patient Characteristics
Characteristics of the RCTs and baseline patient characteristics are summarized in Table 1 and Table S1 in the electronic supplementary material, respectively. In brief, three phase 2 dose-ranging studies and eight phase 3 trials were included in the analysis [15,16,17,18,19,20,21, 24, 27,28,29]. In total, 3244 patients (2.5 µg tiotropium Respimat®, n = 1642; placebo, n = 1602) were included in the analysis. Overall, the duration of treatment ranged from 4 to 52 weeks; mean patient age, from 3.1 to 47.8 years; and mean duration of asthma, from 1.4 to 22.1 years. In all trials, tiotropium Respimat® was added to concomitant ICS therapy with or without other controller medications; the mean dose (standard deviation) of ICS ranged from 228.0 (111.0) to 736.6 (347.9) µg of budesonide or equivalent dose.
Quality Assessment
All trials included in the analysis had a low risk of bias based on the seven domains of bias that were assessed. See Fig. S2 in the electronic supplementary material for details. Seven of the 11 studies had a low risk of bias for all the domains, whereas four studies showed an unclear risk of bias for the selective reporting domains and one trial showed an unclear risk of bias for the blinding of outcome assessment, based on the available information.
Key Outcomes
Peak FEV1
The effect of tiotropium Respimat® on peak FEV1 was evaluated in nine of the 11 trials (Fig. 2) [15,16,17,18, 20, 21, 27,28,29]. In seven trials [16,17,18, 20, 21, 27, 29], statistically significant improvements in peak FEV1 were observed with 2.5 µg tiotropium Respimat® compared with placebo. The greatest improvement was observed in a trial in adults with moderate asthma reported by Kerstjens et al.; after 24 weeks of treatment, the adjusted mean difference in peak FEV1 was 223 mL (95% confidence interval [CI] 185, 262), favoring treatment with 2.5 µg tiotropium Respimat® (p < 0.0001) [20].
Trough FEV1
Trough FEV1 was assessed as a primary or secondary endpoint in 10 of the 11 trials (Fig. 2) [15,16,17,18,19,20,21, 27,28,29]. Since Vrijlandt et al. assessed the efficacy and safety of tiotropium Respimat® in preschool children (aged 1–5 years), spirometric evaluation was possibly excluded for practical reasons [24]. In five trials, statistically significant improvements in trough FEV1 were reported with 2.5 µg tiotropium Respimat® compared with placebo [18, 20, 21, 27, 29]. In line with the results on peak FEV1, the greatest magnitude of change was reported by Kerstjens et al. in adults with moderate asthma; the adjusted mean difference in trough FEV1 was 180 mL (95% CI [138, 221]), favoring treatment with 2.5 µg tiotropium Respimat® versus placebo (p < 0.0001) [20].
Peak and Trough FVC
Overall, peak and trough FVC were assessed as secondary or additional endpoints in six and seven trials, respectively (Fig. 2) [15,16,17, 19,20,21, 29]. Significant improvements in peak and trough FVC were observed with 2.5 µg tiotropium Respimat® in three trials in adults and school-aged children (6–11 years) with moderate asthma [20, 21, 29].
Peak Expiratory Flow
Overall, nine studies investigated PEF (either morning/evening PEF or peak/trough PEF; Fig. 3) [15, 16, 18,19,20,21, 27,28,29]. Among the six studies showing significant improvements in PEF with 2.5 µg tiotropium Respimat® compared with placebo, the greatest improvements were achieved in school-aged children (6–11 years) and adults with mild-to-moderate and moderate asthma, respectively [18, 20, 21].
Asthma Questionnaire Scores
Assessment of asthma control using the ACQ-7 or ACQ-IA was reported for six and two studies, respectively (Fig. 4) [15,16,17,18, 20, 21, 27, 29]. Although ACQ-7/ACQ-IA scores were numerically lower with 2.5 µg tiotropium Respimat® compared with placebo in most of the studies, statistically significant differences were only observed in two studies in adults with moderate asthma [20, 29].
The effect of once-daily 2.5 µg TioR on ACQ-7 scores. *Data presented as adjusted mean difference (SD). **Data presented for ACQ-IA. ACQ-7 Asthma Control Questionnaire 7, ACQ-IA Interviewer-Administered Version of the ACQ, CI confidence interval, MD missing data, SD standard deviation, TioR tiotropium Respimat®
Adverse Events
Asthma, reduction in PEF rate, nasopharyngitis, and respiratory tract infections were among the most commonly reported AEs in the studies included in this analysis (Table 2).
Discussion
In this first systematic review of RCTs assessing the USA-approved dose of tiotropium Respimat® in patients with asthma, once-daily 2.5 µg tiotropium Respimat® significantly improved a number of lung function parameters (peak and trough FEV1 and FVC, and PEF) and PRO measures (ACQ-7 and ACQ-IA scores) compared with placebo and was generally well tolerated. The studies analyzed patients with inadequately controlled asthma at GINA step 2 (low-dose ICS and no other controller medication) [18, 24], step 3 (low-dose ICS plus a LABA or medium-/high-dose ICS) [17, 19,20,21, 27,28,29], or step 4 (medium-/high-dose ICS plus LABA with/without LTRA) [15, 16]. Improvements in peak and trough FEV1 were observed with 2.5 µg tiotropium Respimat® compared with placebo in the majority of studies [16,17,18, 20, 21, 27, 29]. Although the minimal clinically important difference for FEV1 responses in patients with asthma is not well defined, the observed effect sizes in this analysis were comparable to those observed with the addition of LABA to ICS therapy [20, 30,31,32]. Our findings show that 2.5 µg tiotropium Respimat® improved FVC in patients with moderate asthma (adults and children aged 6–11 years), providing a good measure of potential effects of tiotropium on small-airway dysfunction [33].
PEF monitoring is an important tool for measuring changes in airway function, particularly in patients who may not accurately perceive symptom worsening [34]. Indeed, PEF, which is reported as a weekly average of values recorded on a daily basis, may prove a more reliable marker for lung function than FEV1, which is often reported as a single value recorded on a given day in a clinic, outside of the patient’s real-life setting [27]. According to our findings, improvements in PEF (morning and evening measurements) were observed with 2.5 µg tiotropium Respimat® compared with placebo in some studies. Taken together, these findings demonstrate that 2.5 µg tiotropium Respimat® consistently improved lung function across different age groups and asthma severities.
In terms of PROs, the ACQ is a standardized tool that has been observed to be responsive to changes in asthma control in adults with asthma. Although significant differences between 2.5 µg tiotropium Respimat® and placebo were only observed in two studies, overall ACQ scores improved (decreased) and were numerically better with 2.5 µg tiotropium Respimat® in the majority of studies [16, 17, 20, 29]. However, it is important to note that a substantial “placebo effect” could be a confounding factor when interpreting the results of asthma clinical trials, particularly when assessing PROs such as ACQ scores [18]. Participation in a clinical trial likely improved compliance with background treatment (e.g., ICS), which in turn may have improved asthma control regardless of the treatment group. Some studies referred to in this review were 12 weeks long and studies of a longer duration might reveal further differences in clinical outcomes and PROs that may exist in patients with milder disease. Importantly, a minimal clinically important difference of 0.5 has been well established for changes in an individual patient [35]; however, its utility in the measurement of intergroup differences has been questioned [36].
Of note, the National Heart, Lung, and Blood Institute (NHLBI) guidelines have not been updated since 2007 and, therefore, do not capture the current disease landscape or recent therapeutic options [37]. Per GINA guidelines, tiotropium is the recommended treatment option at step 4 (as an add-on to medium-/high-dose ICS and LABA) and at step 5 (as an add-on) [8]. Hence, clinicians should consider results from recent clinical trials and adhere to GINA recommendations [8], which are updated on a yearly basis, when considering treatment options. Interestingly, results from a post hoc analysis of four phase 3 trials, in which the influence of patients’ T2 phenotype on treatment effect was modeled using serum IgE levels and blood eosinophil counts, showed that tiotropium Respimat® improved peak and trough FEV1 in adults with moderate and severe asthma, regardless of T2 phenotype [38]. Although exploratory in nature, these findings suggest that tiotropium can be used without prior phenotyping and therefore can be considered in patients with moderate-to-severe asthma before initiating biologic treatment. If patients continue to have poorly controlled asthma despite tiotropium add-on treatment, or have a diagnosis of atopy, they should be referred to specialists for further investigation and a potential switch to biologics upon appropriate phenotyping.
The safety and tolerability of tiotropium in asthma are well documented. In an expert opinion reviewing 13 published clinical trials comparing tiotropium with placebo or an active control in patients with asthma, the safety of tiotropium was comparable with that of placebo and alternative therapeutic options, including higher doses of ICSs and LABAs [39]. After reviewing the results of long-term trials, Tan et al. concluded that various doses of tiotropium Respimat® were generally well tolerated in patients with asthma, with low rates of discontinuation and extremely rare fatal events [40]. In agreement with previously published evidence, our analysis found that the nature and frequency of AEs and the overall safety profile of 2.5 µg tiotropium Respimat® were comparable to those of placebo [41].
Our systematic review has strengths and limitations that should be considered when interpreting results. Positively, this systematic review was the first to discuss the efficacy and safety of 2.5 µg tiotropium Respimat® in patients with asthma. In addition, most included studies were large phase 3 studies that were pivotal for US FDA approval. Finally, we presented data in this systematic review only for descriptive purposes because heterogeneity in patient population with respect to age group and asthma severity precluded the possibility of performing a statistical analysis (i.e., meta-analysis) of efficacy and safety data.
On the basis of the evidence accumulated from the trials discussed herein, tiotropium is recommended in patients aged at least 6 years in the GINA pocket guide for “Diagnosis and management of difficult-to-treat and severe asthma” [42].
Conclusion
With a wealth of evidence on the efficacy and safety of 2.5 µg tiotropium Respimat®, tiotropium once-daily is an effective add-on treatment to ICS therapy in patients with moderate to severe asthma.
References
Centers for Disease Control and Prevention. National current asthma prevalence. 2016. https://www.cdc.gov/asthma/most_recent_data.htm. Accessed Mar 8, 2019.
Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008–2013. Ann Am Thorac Soc. 2018;15(3):348–56.
Carr TF, Bleecker E. Asthma heterogeneity and severity. World Allergy Organ J. 2016;9(1):41.
Wark PA, Gibson PG. Asthma exacerbations. 3: Pathogenesis. Thorax. 2006;61(10):909–15.
Mathur SK, Viswanathan RK. Relevance of allergy in adult asthma. Curr Allergy Asthma Rep. 2014;14(5):437.
Krishnan V, Diette GB, Rand CS, et al. Mortality in patients hospitalized for asthma exacerbations in the United States. Am J Respir Crit Care Med. 2006;174(6):633–8.
Cabana MD, Kunselman SJ, Nyenhuis SM, Wechsler ME. Researching asthma across the ages: insights from the National Heart, Lung, and Blood Institute’s Asthma Network. J Allergy Clin Immunol. 2014;133(1):27–33.
Global Initiative for Asthma. Pocket guide for asthma management and prevention. Updated 2019. https://ginasthma.org/wp-content/uploads/2019/04/GINA-2019-main-Pocket-Guide-wms.pdf. Accessed Apr 25, 2019.
Kaplan A, Stanbrook M. Must family physicians use spirometry in managing asthma patients? YES. Can Fam Physician. 2010;56(2):126–8.
Chhabra SK. Clinical application of spirometry in asthma: why, when and how often? Lung India. 2015;32(6):635–7.
Masoli M, Weatherall M, Holt S, Beasley R. Moderate dose inhaled corticosteroids plus salmeterol versus higher doses of inhaled corticosteroids in symptomatic asthma. Thorax. 2005;60(9):730–4.
Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170(8):836–44.
Spiriva® Respimat® (tiotropium bromide) inhalation spray, for oral inhalation use. Highlights of Prescribing Information. Boehringer Ingelheim Pharmaceuticals, Inc. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207070Orig1s000lbl.pdf. Accessed Mar 8, 2019.
Spiriva® Respimat® (2.5 microgram, inhalation solution). Summary of Product Characteristics. Boehringer Ingelheim Pharmaceuticals, Inc. https://www.medicines.org.uk/emc/product/407/smpc/print. Accessed Mar 8, 2019.
Szefler SJ, Murphy K, Harper T 3rd, et al. A phase III randomized controlled trial of tiotropium add-on therapy in children with severe symptomatic asthma. J Allergy Clin Immunol. 2017;140(5):1277–87.
Hamelmann E, Bernstein JA, Vandewalker M, et al. A randomised controlled trial of tiotropium in adolescents with severe symptomatic asthma. Eur Respir J. 2017;49(1):1601100.
Hamelmann E, Bateman ED, Vogelberg C, et al. Tiotropium add-on therapy in adolescents with moderate asthma: a 1-year randomized controlled trial. J Allergy Clin Immunol. 2016;138(2):441–450.e8.
Paggiaro P, Halpin DM, Buhl R, et al. The effect of tiotropium in symptomatic asthma despite low- to medium-dose inhaled corticosteroids: a randomized controlled trial. J Allergy Clin Immunol Pract. 2016;4(1):104–113.e2.
Ohta K, Ichinose M, Tohda Y, et al. Long-term once-daily tiotropium Respimat® is well tolerated and maintains efficacy over 52 weeks in patients with symptomatic asthma in Japan: a randomised, placebo-controlled study. PLoS One. 2015;10(4):e0124109.
Kerstjens HA, Casale TB, Bleecker ER, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med. 2015;3(5):367–76.
Vogelberg C, Engel M, Laki I, et al. Tiotropium add-on therapy improves lung function in children with symptomatic moderate asthma. J Allergy Clin Immunol Pract. 2018;6(6):2160–2162.e9.
Kerstjens HA, Engel M, Dahl R, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367(13):1198–207.
Kerstjens HA, Disse B, Schröder-Babo W, et al. Tiotropium improves lung function in patients with severe uncontrolled asthma: a randomized controlled trial. J Allergy Clin Immunol. 2011;128(2):308–14.
Vrijlandt EJLE, El Azzi G, Vandewalker M, et al. Safety and efficacy of tiotropium in children aged 1-5 years with persistent asthmatic symptoms: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2018;6(2):127–37.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41.
Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Vogelberg C, Moroni-Zentgraf P, Leonaviciute-Klimantaviciene M, et al. A randomised dose-ranging study of tiotropium Respimat® in children with symptomatic asthma despite inhaled corticosteroids. Respir Res. 2015;16:20.
Vogelberg C, Engel M, Moroni-Zentgraf P, et al. Tiotropium in asthmatic adolescents symptomatic despite inhaled corticosteroids: a randomised dose-ranging study. Respir Med. 2014;108(9):1268–76.
Beeh KM, Moroni-Zentgraf P, Ablinger O, et al. Tiotropium Respimat® in asthma: a double-blind, randomised, dose-ranging study in adult patients with moderate asthma. Respir Res. 2014;15:61.
Wechsler ME, Yawn BP, Fuhlbrigge AL, et al. Anticholinergic vs long-acting β-agonist in combination with inhaled corticosteroids in black adults with asthma: the BELT randomized clinical trial. JAMA. 2015;314(16):1720–30.
Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363(18):1715–26.
Buhl R, FitzGerald JM, Busse WW. Tiotropium add-on to inhaled corticosteroids versus addition of long-acting β2-agonists for adults with asthma. Respir Med. 2018;143:82–90.
Gibbons WJ, Sharma A, Lougheed D, Macklem PT. Detection of excessive bronchoconstriction in asthma. Am J Respir Crit Care Med. 1996;153(2):582–9.
Callahan KA, Panter TM, Hall TM, Slemmons M. Peak flow monitoring in pediatric asthma management: a clinical practice column submission. J Pediatr Nurs. 2010;25(1):12–7.
Barnes PJ, Casale TB, Dahl R, et al. The Asthma Control Questionnaire as a clinical trial endpoint: past experience and recommendations for future use. Allergy. 2014;69(9):1119–40.
Bateman ED, Esser D, Chirila C, et al. Magnitude of effect of asthma treatments on Asthma Quality of Life Questionnaire and Asthma Control Questionnaire scores: systematic review and network meta-analysis. J Allergy Clin Immunol. 2015;136(4):914–22.
National Heart, Lung, and Blood Institute. Expert Panel Report 3: guidelines for the diagnosis and management of asthma. Full report 2007. https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthgdln_1.pdf. Accessed Mar 8, 2019.
Casale TB, Bateman ED, Vandewalker M, et al. Tiotropium Respimat add-on is efficacious in symptomatic asthma, independent of T2 phenotype. J Allergy Clin Immunol Pract. 2018;6(3):923–935.e9.
Kerstjens HA, O’Byrne PM. Tiotropium for the treatment of asthma: a drug safety evaluation. Expert Opin Drug Saf. 2016;15(8):1115–24.
Tan CK, Say GQ, Geake JB. Long-term safety of tiotropium delivered by Respimat® SoftMist™ Inhaler: patient selection and special considerations. Ther Clin Risk Manag. 2016;12:1433–44.
Dahl R, Engel M, Dusser D, et al. Safety and tolerability of once-daily tiotropium Respimat® as add-on to at least inhaled corticosteroids in adult patients with symptomatic asthma: a pooled safety analysis. Respir Med. 2016;118:102–11.
Global Initiative for Asthma. Diagnosis and management of difficult-to-treat and severe asthma: GINA pocket guide for health professionals. Updated 2018. https://ginasthma.org/wp-content/uploads/2018/11/GINA-SA-FINAL-wms.pdf. Accessed Apr 3, 2019.
Acknowledgements
Funding
Development of the manuscript was funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). The authors received no direct compensation related to the development of the manuscript. BIPI was given the opportunity to review the manuscript for medical and scientific accuracy, as well as intellectual property considerations. As Timothy Craig is a member of the Editorial Board the rapid service fee was waived.
Medical Writing and Editorial Assistance
Writing, editorial support, and formatting assistance was provided by Saurabh Gagangras, PhD, and Maribeth Bogush, MCI, PhD, of Cactus Communications, which was contracted and compensated by BIPI for these services.
Authorship
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE), take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. The sponsor also provided medical, regulatory, legal, and IP review of the final draft manuscript; suggestions were incorporated at the author’s discretion.
Disclosures
Lyndon Mansfield has received research grants from Teva, Pearl, GlaxoSmithKline, Novartis, Amphastar, Aimmune, Cipla, West-Ward, Sanofi, Chiesi, and Lupin. Sy Duong-Quy has nothing to disclose. Timothy Craig has received research grants from Genentech, Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline, Regeneron, and Novartis, and has received traveling grants from and is on the speakers’ bureau for GlaxoSmithKline and Regeneron.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.9334325.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mansfield, L., Duong-Quy, S. & Craig, T. Burden of Asthma and Role of 2.5 µg Tiotropium Respimat® as an Add-On Therapy: A Systematic Review of Phase 2/3 Trials. Adv Ther 36, 2587–2599 (2019). https://doi.org/10.1007/s12325-019-01062-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-01062-w