Abstract
Introduction
Vitamin E is one of the most promising agents for nonalcoholic steatohepatitis (NASH) treatment, and its drug responsiveness may be closely associated with haptoglobin (Hp) genotype. However, its efficacy and safety remain unknown in China. This clinical trial of vitamin E versus placebo for the treatment of nondiabetic patients with nonalcoholic steatohepatitis (VENS) is conducted to evaluate (a) the efficacy and safety of treatment with vitamin E softgel (300 mg/day) determined from standardized histologic scoring of liver biopsies, (b) whether treatment with vitamin E improves biochemical parameters, cytokines, anthropometric parameters, controlled attenuation parameter (CAP), and transient elastography (TE) values determined by Fibroscan and health-related quality of life (SF-36), (c) whether the efficacy of vitamin E treatment is associated with the Hp genotype in nondiabetic adults with NASH.
Methods
VENS is a multicenter, randomized, double-masked, placebo parallel controlled trial to evaluate the efficacy and safety of treatment with vitamin E softgel in nondiabetic adults with NASH versus treatment with placebo in China. Liver biopsies are read by a pathological evaluation committee independently according to the NASH Clinical Research Network (CRN) scoring system. The NAFLD activity score (NAS) represents the sum of scores for steatosis, lobular inflammation, and hepatocyte ballooning. The definition of histologic improvement requires all three of the following criteria to be met: (a) either improvement in NAS by at least 2 points or post-treatment NAS score no higher than 3, (b) at least 1-point improvement in the score for ballooning, and (c) no worsening of fibrosis stages. We plan to recruit 120 biopsy-proven NASH patients from13 centers in China. Participants will be randomly assigned to groups treated with either with vitamin E (100 mg, tid) or placebo for 96 weeks then followed by 24 weeks of post-treatment observation. Biochemical parameters, cytokines, anthropometric parameters, CAP and TE values, Hp genotype, and several questionnaires will be collected as per the schedule. This protocol was approved by the Ethics Committee of Hangzhou Normal University Affiliated Hospital to ensure patients safety, and R&G Pharmastudies Co., Ltd. was established for monitoring the accumulated interim data to review efficacy and quality of data collection and overall study management.
Results
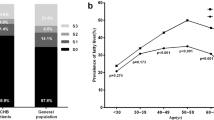
As a preliminary study, a mobile phone application (app) for lifestyle modification and database recording (http://laiyivens.365hy.com) was exploited for every participant. The percentage of NAFLD patients with Hp 2-2 allele is much higher than that of Western patients (65.71% vs 36%, respectively), which suggests that the Chinese benefit more from vitamin E treatment.
Conclusion
VENS is the first randomized controlled trial (RCT) to evaluate the efficacy of Vitamin E in treating nondiabetic NASH patients in China.
Trial Registration
This study registered at https://clinicaltrials.gov (registration number: NCT02962297).
Funding
Zhejiang Medicine Co., Ltd.



Similar content being viewed by others
References
Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47–64.
Ballestri S, Zona S, Targher G, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:936–44.
Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis. 2015;47:181–90.
Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65:589–600.
Targher G, Bertolini L, Rodella S, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008;51:444–50.
Italian Association for the Study of the Liver (AISF). AISF position paper on nonalcoholic fatty liver disease. (NAFLD): updates and future directions. Dig Liver Dis. 2017;49:471–83.
Ballestri S, Nascimbeni F, Romagnoli D, Lonardo A. The independent predictors of non-alcoholic steatohepatitis and its individual histological features. Insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatol Res. 2016;46:1074–87.
Mantovani A, Targher G. Type 2 diabetes mellitus and risk of hepatocellular carcinoma: spotlight on nonalcoholic fatty liver disease. Ann Transl Med. 2017;5:270.
Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73.
Ballestri S, Nascimbeni F, Romagnoli D, Baldelli E, Lonardo A. The role of nuclear receptors in the pathophysiology, natural course, and drug treatment of NAFLD in humans. Adv Ther. 2016;33:291–319.
Lonardo A, Nascimbeni F, Maurantonio M, Marrazzo A, Rinaldi L, Adinolfi LE. Nonalcoholic fatty liver disease: evolving paradigms. World J Gastroenterol. 2017;23:6571–92.
Brandi G, De Lorenzo S, Candela M, et al. Microbiota, NASH, HCC and the potential role of probiotics. Carcinogenesis. 2017;38:231–40.
Zoller H, Tilg H. Nonalcoholic fatty liver disease and hepatocellular carcinoma. Metabolism. 2016;65:1151–60.
Xun YH, Fan JG, Zang GQ, et al. Suboptimal performance of simple noninvasive tests for advanced fibrosis in Chinese patients with nonalcoholic fatty liver disease. J Dig Dis. 2012;13:588–95.
Shen F, Zheng RD, Shi JP, et al. Impact of skin capsular distance on the performance of controlled attenuation parameter in patients with chronic liver disease. Liver Int. 2015;35:2392–400.
Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–90.
Barb D, Portillo-Sanchez P, Cusi K. Pharmacological management of nonalcoholic fatty liver disease. Metabolism. 2016;65:1183–95.
Ratziu V, Goodman Z, Sanyal A. Current efforts and trends in the treatment of NASH. J Hepatol. 2015;62:S65–75.
Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1497–502.
Chalasani N, Gorski JC, Asghar MS, et al. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology. 2003;37:544–50.
Karimian G, Kirschbaum M, Veldhuis ZJ, Bomfati F, Porte RJ, Lisman T. Vitamin E attenuates the progression of non-alcoholic fatty liver disease caused by partial hepatectomy in mice. PLoS One. 2015;10:e0143121.
Soden JS, Devereaux MW, Haas JE, et al. Subcutaneous vitamin E ameliorates liver injury in an in vivo model of steatocholestasis. Hepatology. 2007;46:485–95.
Sokol RJ, McKim JM Jr, Goff MC, et al. Vitamin E reduces oxidant injury to mitochondria and the hepatotoxicity of taurochenodeoxycholic acid in the rat. Gastroenterology. 1998;114:164–74.
Kim GH, Chung JW, Lee JH, et al. Effect of vitamin E in nonalcoholic fatty liver disease with metabolic syndrome: a propensity score-matched cohort study. Clin Mol Hepatol. 2015;21:379–86.
Fukui A, Kawabe N, Hashimoto S, et al. Vitamin E reduces liver stiffness in nonalcoholic fatty liver disease. World J Hepatol. 2015;7:2749–56.
Sumida Y, Naito Y, Tanaka S, et al. Long-term (>=2 yr) efficacy of vitamin E for non-alcoholic steatohepatitis. Hepatogastroenterology. 2013;60:1445–50.
Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85.
Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–68.
Bell LN, Wang J, Muralidharan S, et al. Relationship between adipose tissue insulin resistance and liver histology in nonalcoholic steatohepatitis: a pioglitazone versus vitamin E versus placebo for the treatment of nondiabetic patients with nonalcoholic steatohepatitis trial follow-up study. Hepatology. 2012;56:1311–8.
Bowman BH, Kurosky A. Haptoglobin: the evolutionary product of duplication, unequal crossing over, and point mutation. Adv Hum Genet. 1982;12(189–261):453–4.
Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem. 1996;42:1589–600.
Melamed-Frank M, Lache O, Enav BI, et al. Structure-function analysis of the antioxidant properties of haptoglobin. Blood. 2001;98:3693–8.
Bamm VV, Tsemakhovich VA, Shaklai M, Shaklai N. Haptoglobin phenotypes differ in their ability to inhibit heme transfer from hemoglobin to LDL. Biochemistry. 2004;43:3899–906.
Vardi M, Levy AP. Is it time to screen for the haptoglobin genotype to assess the cardiovascular risk profile and vitamin E therapy responsiveness in patients with diabetes? Curr Diab Rep. 2012;12:274–9.
Milman U, Blum S, Shapira C, et al. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: a prospective double-blinded clinical trial. Arterioscler Thromb Vasc Biol. 2008;28:341–7.
Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:56–65.
Blum S, Vardi M, Levy NS, Miller-Lotan R, Levy AP. The effect of vitamin E supplementation on cardiovascular risk in diabetic individuals with different haptoglobin phenotypes. Atherosclerosis. 2010;211:25–7.
Orchard TJ, Sun W, Cleary PA, et al. Haptoglobin genotype and the rate of renal function decline in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes. 2013;62:3218–23.
Costacou T, Ferrell RE, Ellis D, Orchard TJ. Haptoglobin genotype and renal function decline in type 1 diabetes. Diabetes. 2009;58:2904–9.
Koch W, Latz W, Eichinger M, et al. Genotyping of the common haptoglobin Hp 1/2 polymorphism based on PCR. Clin Chem. 2002;48:1377–82.
Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21.
Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther. 2017;34:1291–326.
Awadallah S, Hamad M. The prevalence of type II diabetes mellitus is haptoglobin phenotype-independent. Cytobios. 2000;101:145–50.
Sun Yongye, Ma Aiguo, Li Yong, Han Xiuxia, Wang Qiuzhen, Liang Hui. Vitamin E supplementation protects erythrocyte membranes from oxidative stress in healthy Chinese middle-aged and elderly people. Nutr Res. 2012;32:328–34.
Acknowledgements
Funding
Vitamin E used in this study and the article processing charges were sponsored by Zhejiang Medicine Co., Ltd. Statistical analysis is supported by the School of Public Health Nanjing Medical University, Department of Biostatistics. We thank Hao yu (PhD) from the Nanjing Medical University statistical team for his contributions to our study design. Clinical data management is provided by R&G Pharma Studies (China) Co., Ltd.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. The authors, individually and collectively, are responsible for all content and editorial decisions and received no payment from any of the above companies directly or indirectly (through a third party) related to this publication. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Disclosures
Shufei Zang, Jin Chen, Yu Song, Bingyuan Wang, and Junping Shi declare that they have nothing to disclose. Lang Bai is a leader of one of the clinical research subcenters. Jinjun Chen is a leader of one of the clinical research subcenters. Xiaoling Chi is a leader of one of the clinical research subcenters. Fangping He is a leader of one of the clinical research subcenters. Huiping Sheng is a leader of one of the clinical research subcenters. Jing Wang is a leader of one of the clinical research subcenters. Wen Xie is a leader of one of the clinical research subcenters. Yongfeng Yang is a leader of one of the clinical research subcenters. Jing Zhang is a leader of one of the clinical research subcenters. Minghua Zheng is a leader of one of the clinical research subcenters. Zhengsheng Zou is a leader of one of the clinical research subcenters. Shilong Xie is an employee of Zhejiang Medicine Co., Ltd Hangzhou Branch.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study. Prior to the start of the trial, the detailed protocol and information of experimental drugs, informed consent, and other necessary materials were submitted to the ethics committee for review and were approved. During the trial, if any changes are required to this protocol or informed consent, permission should be given again. In addition, any adverse events monitored in the trial should be reported regularly to the ethics committee during the progress of the trial.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/4CFCF060158096D0.
Rights and permissions
About this article
Cite this article
Zang, S., Chen, J., Song, Y. et al. Haptoglobin Genotype and Vitamin E Versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis in China: A Multicenter, Randomized, Placebo-Controlled Trial Design. Adv Ther 35, 218–231 (2018). https://doi.org/10.1007/s12325-018-0670-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-018-0670-8