Abstract
Introduction
The present study was conducted as a pilot to compare the therapeutic effects and the potential side effects of oral Megestrol acetate and Letrozole in the treatment of simple hyperplasia in perimenopausal women.
Methods
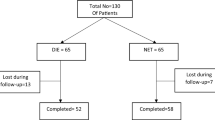
The participants of this randomized clinical trial consisted of two groups of 25 women aged 44–50 presenting with abnormal uterine bleeding diagnosed with simple endometrial hyperplasia without cytologic atypia confirmed by transvaginal ultrasonography and biopsy. The first group received 40-mg doses of Megestrol acetate for 2 weeks per month for a total period of 2 months. The second group received 2.5-mg daily doses of Letrozole for a total period of 2 months. The differences in terms of quantitative measurements were analyzed using the independent two-sample t test and the paired t test. To compare the two groups in terms of the distribution of the categorical variables, Pearson’s Chi square and Fisher’s Exact tests were used at the significance level of 0.05 by Stata-9.2.
Results
Although the intervention led to significant improvements in both groups (P < .001), there was no difference between the groups in terms of accomplishing resolution (P = .74) [seven (28%) patients in the Letrozole group and five (20%) in the Megestrol group], while two patients in the Letrozole group and nine in the Megestrol group suffered from side effects, suggesting significantly lower side effects in the Letrozole group (P = .02).
Conclusion
Letrozole and Megestrol acetate seem to have similar effects on the treatment of simple endometrial hyperplasia, the only difference being that Letrozole presents fewer side effects than Megestrol acetate in patients with this condition.
Funding
Abnormal Uterine Bleeding Research Center of Semnan University of Medical Sciences, Semnan, Iran.
Trial Registration: IRCT2015031011504N5
Similar content being viewed by others
References
Tasci Y, Polat OG, Ozdogan S, Karcaaltincaba D, Seckin L, Erkaya S. Comparison of the efficacy of micronized progesterone and lynestrenol in treatment of simple endometrial hyperplasia without atypia. Arch Gynecol Obstet. 2014;290(1):83–6.
Armstrong AJ, Hurd WW, Elguero S, Barker NM, Zanotti KM. Diagnosis and management of endometrial hyperplasia. J Minim Invasive Gynecol. 2012;19(5):562–71.
Montgomery BE, Daum GS, Dunton CJ. Endometrial hyperplasia: a review. Obstet Gynecol Surv. 2004;59(5):368–78.
Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia.A long-term study of “untreated” hyperplasia in 170 patients. Obstet Gynecol Surv. 1986;41(1):58–61.
Trimble CL, Method M, Leitao M, et al. Management of endometrial precancers. Obstet Gynecol. 2012;120(5):1160.
Haimovich S, Checa MA, Mancebo G, Fusté P, Carreras R. Treatment of endometrial hyperplasia without atypia in peri-and postmenopausal women with a levonorgestrel intrauterine device. Menopause. 2008;15(5):1002–7.
Crosignani P, Luciano A, Ray A, Bergqvist A. Subcutaneous depot medroxyprogesterone acetate versus leuprolide acetate in the treatment of endometriosis-associated pain. Hum Reprod. 2006;21(1):248–56.
Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leuprolide acetate in the treatment of endometriosis-associated pain. Fertil Steril. 2006;85(2):314–25.
Ismail MT, Fahmy DM, Elshmaa NS. Efficacy of levonorgestrel-releasing intrauterine system versus oral progestins in treatment of simple endometrial hyperplasia without atypia. Reprod Sci. 2013;20(1):45–50.
Amezcua CA, Lu JJ, Felix JC, Stanczyk FZ, Zheng W. Apoptosis may be an early event of progestin therapy for endometrial hyperplasia. Gynecol Oncol. 2000;79(2):169–76.
Ferenczy A, Gelfand M. The biologic significance of cytologic atypia in progestogen-treated endometrial hyperplasia. Am J Obstet Gynecol. 1989;160(1):126–31.
Randall TC, Kurman RJ. Progestin treatment of atypical hyperplasia and well-differentiated carcinoma of the endometrium in women under age 40. Obstet Gynecol. 1997;90(3):434–40.
Moradan S, Omidvar V. Desogestrel + ethinylestradiol versus levonorgestrel + ethinylestradiol. Which one has better affect on acne, hirsutism, and weight change? Saudi medical journal. 2011;32(1):23–6.
Li H, Chen X, Qiao J. Letrozole as primary therapy for endometrial hyperplasia in young women. Int J Gynecol Obstet. 2008;100(1):10–2.
Gupta S. A Comprehensive Textbook of Obstetrics and Gynecology. Jaypee Brothers Medical Publishers. 2011; 1th Edition 268.
Wheeler DT, Bristow RE, Kurman RJ. Histologic alterations in endometrial hyperplasia and well- differentiated carcinoma treated with progestins. Am J Surg Pathol. 2007;31(7):988–98.
Tabatabaie A, Karimi ZM, Dehghani-Tafti M, Miratashi-Yazdi A, Teimoori S, Dehghani A. Comparing letrozole with medroxyprogesterone acetate (MPA) as hormonal therapy for simple endometrial hyperplasia without atypia in adult and middle-aged women. Eur J Gynaecol Oncol. 2012;34(6):552–5.
Lähteenmäki P, Rauramo I, Backman T. The levonorgestrel intrauterine system in contraception. Steroids. 2000;65(10):693–7.
Barker L, Brand I, Crawford S. Sustained effect of the aromatase inhibitors anastrozole and letrozole on endometrial thickness in patients with endometrial hyperplasia and endometrial carcinoma. Curr Med Res Opin. 2009;25(5):1105–9.
Espindola D, Kennedy KA, Fischer EG. Management of abnormal uterine bleeding and the pathology of endometrial hyperplasia. Obstet Gynecol Clin North Am. 2007;34(4):717–37.
Emons G, Beckmann M, Schmidt D, Mallmann P. New WHO classification of endometrial hyperplasias. Geburtshilfe Frauenheilkd. 2015;75(2):135–6.
Acknowledgements
This study was supported and funded by the Abnormal Uterine Bleeding Research Center of Semnan University of Medical Sciences as a thesis project for achieving specialty degree in the field of gynecology and obstetrics with registry clinical trials code IRCT2015031011504N5. No funding or sponsorship was received for article processing charges. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Sanam Moradan, Niaz Nikkhah and Majid Mirmohammadkhanai declare no conflicts of interest.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content To view enhanced content for this article go to www.medengine.com/Redeem/F4F7F06079BD157D.
Rights and permissions
About this article
Cite this article
Moradan, S., Nikkhah, N. & Mirmohammadkhanai, M. Comparing the Administration of Letrozole and Megestrol Acetate in the Treatment of Women with Simple Endometrial Hyperplasia without Atypia: A Randomized Clinical Trial. Adv Ther 34, 1211–1220 (2017). https://doi.org/10.1007/s12325-017-0509-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-017-0509-8