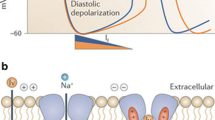
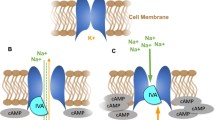
Abstract
Heart failure has seen a number of therapeutic advances in recent years. Despite this, heart failure is still related to increasing rates of morbidity, repeated hospitalizations, and mortality. Ivabradine is a recent treatment option for heart failure. It has a mode of action that includes reduction in heart rate, and leads to improvement in outcomes related to heart failure mortality and morbidity, as demonstrated by the results of the SHIFT trial in patients with systolic heart failure, functional classes II and III on the New York Heart Association classification, and left ventricular ejection fraction ≤35%. These results are intriguing since many heart failure drugs reduce heart rate without such benefits, or with quite different effects, making it more difficult to understand the novelty of ivabradine in this setting. Many of the drugs used in heart failure modify heart rate, but most have other pathophysiological effects beyond their chronotropic action, which affect their efficacy in preventing morbidity and mortality outcomes. For instance, heart rate reduction at rest or exercise with ivabradine prolongs diastolic perfusion time, improves coronary blood flow, and increases exercise capacity. Another major difference is the increase in stroke volume observed with ivabradine, which may underlie its beneficial cardiac effects. Finally, there is mounting evidence from both preclinical and clinical studies that ivabradine has an anti-remodeling effect, improving left ventricular structures and functions. All together, these mechanisms have a positive impact on the prognosis of ivabradine-treated patients with heart failure, making a compelling argument for use of ivabradine in combination with other treatments.
Funding
Servier.
Similar content being viewed by others
References
The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316(23):1429–35.
Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327(10):669–77.
Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–17.
CIBIS Investigators and Committees. A randomized trial of beta-blockade in heart failure. The Cardiac Insufficiency Bisoprolol Study (CIBIS). Circulation. 1994;90(4):1765–73.
Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334(21):1349–55.
Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350(21):2140–50.
Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539–49.
Stewart S, Ekman I, Ekman T, Oden A, Rosengren A. Population impact of heart failure and the most common forms of cancer: a study of 1162309 hospital cases in Sweden (1988 to 2004). Circ Cardiovasc Qual Outcomes. 2010;3(6):573–80.
McMurray JJ, Pfeffer MA. Heart failure. Lancet. 2005;365(9474):1877–89.
Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled trial. Lancet. 2010;376(9744):875–85.
Borer JS, Bohm M, Ford I, et al. Effect of ivabradine on recurrent hospitalization for worsening heart failure in patients with chronic systolic heart failure: the SHIFT Study. Eur Heart J. 2012;33(22):2813–20.
Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111(21):2837–49.
Packer M. How should physicians view heart failure? The philosophical and physiological evolution of three conceptual models of the disease. Am J Cardiol. 1993;71(9):3C–11C.
Sandler H, Dodge HT. Left ventricular tension and stress in man. Circ Res. 1963;13:91–104.
Hood WP Jr, Rackley CE, Rolett EL. Wall stress in the normal and hypertrophied human left ventricle. Am J Cardiol. 1968;22(4):550–8.
Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56(1):56–64.
Meerson FZ. On the mechanism of compensatory hyperfunction and insufficiency of the heart. Cor Vasa. 1961;31:61–77.
Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322(22):1561–6.
Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114(5):345–52.
Gunther S, Grossman W. Determinants of ventricular function in pressure-overload hypertrophy in man. Circulation. 1979;59(4):679–88.
Huber D, Grimm J, Koch R, Krayenbuehl HP. Determinants of ejection performance in aortic stenosis. Circulation. 1981;64(1):126–34.
Krayenbuehl HP, Hess OM, Ritter M, Monrad ES, Hoppeler H. Left ventricular systolic function in aortic stenosis. Eur Heart J. 1988;9(Suppl):E19–23.
Katz AM. Maladaptive growth in the failing heart: the cardiomyopathy of overload. Cardiovasc Drugs Ther. 2002;16(3):245–9.
Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367(9507):356–67.
Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348(20):2007–18.
Klabunde RE. Cardiovascular Physiology Concepts. 2nd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2012.
Pereira-Barretto AC. Most heart failure patients die from pump failure. Am J Cardiovasc Drugs. 2015. (In press).
Heusch G, Libby P, Gersh B, et al. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. 2014;383(9932):1933–43.
Heusch G. Heart rate and heart failure. Not a simple relationship. Circ J. 2011;75(2):229–36.
McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14(8):803–69.
Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28(20):2539–50.
Komajda M, Lam CS. Heart failure with preserved ejection fraction: a clinical dilemma. Eur Heart J. 2014;35(16):1022–32.
Komamura K. Similarities and differences between the pathogenesis and pathophysiology of diastolic and systolic heart failure. Cardiol Res Pract. 2013;2013:824135.
Chatterjee K, Massie B. Systolic and diastolic heart failure: differences and similarities. J Card Fail. 2007;13(7):569–76.
Dobre D, Borer JS, Fox K, et al. Heart rate: a prognostic factor and therapeutic target in chronic heart failure. The distinct roles of drugs with heart rate-lowering properties. Eur J Heart Fail. 2014;16(1):76–85.
Whitbeck MG, Charnigo RJ, Khairy P, et al. Increased mortality among patients taking digoxin—analysis from the AFFIRM Study. Eur Heart J. 2013;34(20):1481–8.
Freeman JV, Yang J, Sung SH, Hlatky MA, Go AS. Effectiveness and safety of digoxin among contemporary adults with incident systolic heart failure. Circ Cardiovasc Qual Outcomes. 2013;6(5):525–33.
DiFrancesco D. The contribution of the ‘pacemaker’ current (if) to generation of spontaneous activity in rabbit sino-atrial node myocytes. J Physiol. 1991;43423-40.
Meiler SE, Boudoulas H, Unverferth DV, Leier CV. Diastolic time in congestive heart failure. Am Heart J. 1987;114(5):1192–8.
Colin P, Ghaleh B, Monnet X, et al. Contributions of heart rate and contractility to myocardial oxygen balance during exercise. Am J Physiol Heart Circ Physiol. 2003;284(2):H676–82.
Colin P, Ghaleh B, Hittinger L, et al. Differential effects of heart rate reduction and beta-blockade on left ventricular relaxation during exercise. Am J Physiol Heart Circ Physiol. 2002;282(2):H672–9.
Custodis F, Schirmer SH, Baumhakel M, Heusch G, Bohm M, Laufs U. Vascular pathophysiology in response to increased heart rate. J Am Coll Cardiol. 2010;56(24):1973–83.
Bache RJ, Cobb FR. Effect of maximal coronary vasodilation on transmural myocardial perfusion during tachycardia in the awake dog. Circ Res. 1977;41(5):648–53.
Ferro G, Duilio C, Spinelli L, Liucci GA, Mazza F, Indolfi C. Relation between diastolic perfusion time and coronary artery stenosis during stress-induced myocardial ischemia. Circulation. 1995;92(3):342–7.
Ferro G, Duilio C, Spinelli L, Spadafora M, Guarnaccia F, Condorelli M. Effects of beta blockade on the relation between heart rate and ventricular diastolic perfusion time during exercise in systemic hypertension. Am J Cardiol. 1991;68(10):1101–3.
Ferro G, Spinelli L, Duilio C, Spadafora M, Guarnaccia F, Condorelli M. Diastolic perfusion time at ischemic threshold in patients with stress-induced ischemia. Circulation. 1991;84(1):49–56.
Skalidis EI, Hamilos MI, Chlouverakis G, Zacharis EA, Vardas PE. Ivabradine improves coronary flow reserve in patients with stable coronary artery disease. Atherosclerosis. 2011;215(1):160–5.
Dedkov EI, Zheng W, Christensen LP, Weiss RM, Mahlberg-Gaudin F, Tomanek RJ. Preservation of coronary reserve by ivabradine-induced reduction in heart rate in infarcted rats is associated with decrease in perivascular collagen. Am J Physiol Heart Circ Physiol. 2007;293(1):H590–8.
Heusch G. Heart rate in the pathophysiology of coronary blood flow and myocardial ischaemia: benefit from selective bradycardic agents. Br J Pharmacol. 2008;153(8):1589–601.
Monnet X, Ghaleh B, Colin P, de Curzon OP, Giudicelli JF, Berdeaux A. Effects of heart rate reduction with ivabradine on exercise-induced myocardial ischemia and stunning. J Pharmacol Exp Ther. 2001;299(3):1133–9.
Monnet X, Colin P, Ghaleh B, Hittinger L, Giudicelli JF, Berdeaux A. Heart rate reduction during exercise-induced myocardial ischaemia and stunning. Eur Heart J. 2004;25(7):579–86.
Heusch G. Pleiotropic action(s) of the bradycardic agent ivabradine: cardiovascular protection beyond heart rate reduction. Br J Pharmacol. 2008;155(7):970–1.
Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97(3):282–9.
Ceconi C, Cargnoni A, Francolini G, Parinello G, Ferrari R. Heart rate reduction with ivabradine improves energy metabolism and mechanical function of isolated ischaemic rabbit heart. Cardiovasc Res. 2009;84(1):72–82.
Komajda M, Hanon O, Hochadel M, et al. Contemporary management of octogenarians hospitalized for heart failure in Europe: Euro Heart Failure Survey II. Eur Heart J. 2009;30(4):478–86.
Volterrani M, Cice G, Caminiti G, et al. Effect of Carvedilol, Ivabradine or their combination on exercise capacity in patients with heart failure (the CARVIVA HF trial). Int J Cardiol. 2011;151(2):218–24.
Sarullo FM, Fazio G, Puccio D, et al. Impact of “off-label” use of ivabradine on exercise capacity, gas exchange, functional class, quality of life, and neurohormonal modulation in patients with ischemic chronic heart failure. J Cardiovasc Pharmacol Ther. 2010;15(4):349–55.
Mulder P, Barbier S, Chagraoui A, et al. Long-term heart rate reduction induced by the selective I(f) current inhibitor ivabradine improves left ventricular function and intrinsic myocardial structure in congestive heart failure. Circulation. 2004;109(13):1674–9.
Dillinger JG, aher V, itale C, et al. Impact of ivabradine on central aortic blood pressure and myocardial perfusion in patients with stable coronary artery disease. Hypertension. 2015. (In press).
De Ferrari GM, Mazzuero A, Agnesina L, et al. Favourable effects of heart rate reduction with intravenous administration of ivabradine in patients with advanced heart failure. Eur J Heart Fail. 2008;10(6):550–5.
Tardif JC, O’Meara E, Komajda M, et al. Effects of selective heart rate reduction with ivabradine on left ventricular remodelling and function: results from the SHIFT echocardiography substudy. Eur Heart J. 2011;32(20):2507–15.
Becher PM, Lindner D, Miteva K, et al. Role of heart rate reduction in the prevention of experimental heart failure: comparison between If-channel blockade and beta-receptor blockade. Hypertension. 2012;59(5):949–57.
Mellin V, Bauer F, Richard V, et al. Short-term heart rate reduction induced by ivabradine improves systolic and diastolic cardiac functions in post-infarcted rats with established chronic heart failure. Abstract 2441. Eur Heart J. 2007;28 (suppl):388.
Reil JC, Tardif JC, Ford I, et al. Selective heart rate reduction with ivabradine unloads the left ventricle in heart failure patients. J Am Coll Cardiol. 2013;62(21):1977–85.
Bagriy AE, Schukina EV, Samoilova OV, et al. Addition of ivabradine to beta-blocker improves exercise capacity in systolic heart failure patients in a prospective, open-label study. Adv Ther. 2015;32(2):108–19.
Zagidullin NS, Zulkarneev RH, Travnikova EO, Zagidullin SZ. Comparison of ivabradine and metoprolol tartrate impact on the heart variability in patients with angina pectoris. Cardiovascular System. 2014;2:9.
Speranza L, Franceschelli S, Riccioni G. The biological effects of ivabradine in cardiovascular disease. Molecules. 2012;17(5):4924–35.
Sabbah HN, Gupta R, Wang M, et al. Heart rate reduction with ivabradine reduces activation of the renin–angiotensin–aldosterone system in dogs with chronic heart failure. J Am Coll Cardiol. 2011;57(17):E197.
Milliez P, Messaoudi S, Nehme J, Rodriguez C, Samuel JL, Delcayre C. Beneficial effects of delayed ivabradine treatment on cardiac anatomical and electrical remodeling in rat severe chronic heart failure. Am J Physiol Heart Circ Physiol. 2009;296:H435–41.
Ceconi C, Comini L, Suffredini S, et al. Heart rate reduction with ivabradine prevents the global phenotype of left ventricular remodeling. Am J Physiol Heart Circ Physiol. 2011;300(1):H366–73.
Fang Y, Debunne M, Vercauteren M, et al. Heart rate reduction induced by the if current inhibitor ivabradine improves diastolic function and attenuates cardiac tissue hypoxia. J Cardiovasc Pharmacol. 2012;59(3):260–7.
Christensen LP, Zhang RL, Zheng W, et al. Postmyocardial infarction remodeling and coronary reserve: effects of ivabradine and beta blockade therapy. Am J Physiol Heart Circ Physiol. 2009;297(1):H322–30.
Suffredini S, Stillitano F, Comini L, et al. Long-term treatment with ivabradine in post-myocardial infarcted rats counteracts f-channel overexpression. Br J Pharmacol. 2012;165(5):1457–66.
Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. On behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35(3):569–82.
Solomon SD, Anavekar N, Skali H, et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112(24):3738–44.
Kleinbongard P, Gedik N, Witting P, Freedman B, Klocker N, Heusch G. Pleiotropic, heart rate-independent cardioprotection by ivabradine. Br J Pharmacol. 2015;172(17):4380–90.
Gerbaud E, Montaudon M, Chasseriaud W, et al. Effect of ivabradine on left ventricular remodelling after reperfused myocardial infarction: a pilot study. Arch Cardiovasc Dis. 2014;107(1):33–41.
Vercauteren M, Favre J, Mulder P, et al. Protection of endothelial function by long-term heart rate reduction induced by ivabradine in a rat model of chronic heart failure. P468. Eur Heart J. 2007;28(suppl):48.
Gupta RC, Wang M, Ilsar I, et al. Heart rate reduction with ivabradine improves sarcoplasmic reticulum calcium cycling in left ventricular myocardium of dogs with moderate heart failure. J Am Coll Cardiol. 2011;57(11):E323.
Remme WJ, Riegger G, Hildebrandt P, et al. The benefits of early combination treatment of carvedilol and an ACE-inhibitor in mild Heart Failure and left ventricular systolic dysfunction. The carvedilol and ACE-inhibitor remodelling mild Heart Failure evaluation trial (CARMEN). Cardiovasc Drugs Ther. 2004;18(1):57–66.
Jondeau G, Böhm M, Tavazzi L, et al. Hemodynamic effects of ivabradine, an agent that reduces heart rate, in patients with moderate to severe systolic heart failure receiving beta-blockers. Arch Mal Coeur Vaiss. 2008;101Abstract.
Busseuil D, Shi Y, Mecteau M, et al. Heart rate reduction by ivabradine reduces diastolic dysfunction and cardiac fibrosis. Cardiology. 2010;117(3):234–42.
Navaratnarajah M, Ibrahim M, Siedlecka U, et al. Influence of ivabradine on reverse remodelling during mechanical unloading. Cardiovasc Res. 2013;97(2):230–9.
Ulu N, Henning RH, Goris M, Schoemaker RG, Van Gilst WH. Effects of ivabradine and metoprolol on cardiac angiogenesis and endothelial dysfunction in rats with heart failure. J Cardiovasc Pharmacol. 2009;53(1):9–17.
Ciobotaru V, Heimburger M, Louedec L, et al. Effect of long-term heart rate reduction by If-current inhibition on pressure-overload-induced heart failure in rats. J Pharmacol Exp Ther. 2007;324(1):43–9.
Acknowledgments
The article processing charges and the open access fee for this publication were funded by Laboratoires Servier, Brazil, an incorporated company of Servier. The named author meets the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, takes responsibility for the integrity of the work as a whole, and has given final approval for the version to be published.
Disclosures
The author has no relevant affiliations or financial involvement with any organization or entity in conflict with the subject matter or materials discussed in the manuscript.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by the author.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pereira-Barretto, A.C. Cardiac and Hemodynamic Benefits: Mode of Action of Ivabradine in Heart Failure. Adv Ther 32, 906–919 (2015). https://doi.org/10.1007/s12325-015-0257-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-015-0257-6