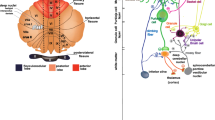
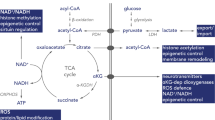
Abstract
Cells configure their metabolism in a synchronized and timely manner to meet their energy demands throughout development and adulthood. Transitions of developmental stages are coupled to metabolic shifts, such that glycolysis is highly active during cell proliferation, whereas oxidative phosphorylation prevails in postmitotic states. In the cerebellum, metabolic transitions are remarkable given its protracted developmental timelines. Such distinctive feature, along with its high neuronal density and metabolic demands, make the cerebellum highly vulnerable to metabolic insults. Despite the expansion of metabolomic approaches to uncover biological mechanisms, little is known about the role of metabolism on cerebellar development and maintenance. To illuminate the intricate connections between metabolism, physiology, and cerebellar disorders, we examined here the impact of metabolism on cerebellar growth, maturation, and adulthood through the lens of inborn errors of metabolism.







Similar content being viewed by others
References
Howarth C, Gleeson P, Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab. 2012;32(7):1222–32.
Howarth C, Peppiatt-Wildman CM, Attwell D. The energy use associated with neural computation in the cerebellum. J Cereb Blood Flow Metab. 2010;30(2):403–14.
Leto K, Arancillo M, Becker EB, Buffo A, Chiang C, Ding B, Dobyns WB, Dusart I, Haldipur P, Hatten ME, Hoshino M, Joyner AL, Kano M, Kilpatrick DL, Koibuchi N, Marino S, Martinez S, Millen KJ, Millner TO, Miyata T, Parmigiani E, Schilling K, Sekerkova G, Sillitoe RV, Sotelo C, Uesaka N, Wefers A, Wingate RJ, Hawkes R. Consensus paper: cerebellar development. Cerebellum. 2016;15(6):789–828.
Tech K, Gershon TR. Energy metabolism in neurodevelopment and medulloblastoma. Transl Pediatr. 2015;4(1):12–9.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33.
Gershon TR, Crowther AJ, Tikunov A, Garcia I, Annis R, Yuan H, Miller CR, Macdonald J, Olson J, Deshmukh M. Hexokinase-2-mediated aerobic glycolysis is integral to cerebellar neurogenesis and pathogenesis of medulloblastoma. Cancer Metab. 2013;1(1):2.
Ferreira CR, van Karnebeek CDM, Vockley J, Blau N. A proposed nosology of inborn errors of metabolism. Genet Med. 2019;21(1):102–6.
Kaminiow K, Rygula I, Paprocka J. Ataxia in neurometabolic disorders. Metabolites. 2022;13(1):47.
Ferreira CR, Rahman S, Keller M, Zschocke J, Group IA. An international classification of inherited metabolic disorders (ICIMD). J Inherit Metab Dis. 2021;44(1):164–77.
Saudubray JM, Garcia-Cazorla A. An overview of inborn errors of metabolism affecting the brain: from neurodevelopment to neurodegenerative disorders. Dialogues Clin Neurosci. 2018;20(4):301–25.
Blaser SI, Steinlin M, Al-Maawali A, Yoon G. The pediatric cerebellum in inherited neurodegenerative disorders: a pattern-recognition approach. Neuroimaging Clin N Am. 2016;26(3):373–416.
Poretti A, Boltshauser E. Terminology in morphological anomalies of the cerebellum does matter. Cerebellum Ataxias. 2015;2:8.
Steinlin M, Blaser S, Boltshauser E. Cerebellar involvement in metabolic disorders: a pattern-recognition approach. Neuroradiology. 1998;40(6):347–54.
Eunson LH, Rea R, Zuberi SM, Youroukos S, Panayiotopoulos CP, Liguori R, Avoni P, McWilliam RC, Stephenson JB, Hanna MG, Kullmann DM, Spauschus A. Clinical, genetic, and expression studies of mutations in the potassium channel gene KCNA1 reveal new phenotypic variability. Ann Neurol. 2000;48(4):647–56.
Glaudemans B, van der Wijst J, Scola RH, Lorenzoni PJ, Heister A, van der Kemp AW, Knoers NV, Hoenderop JG, Bindels RJ. A missense mutation in the Kv1.1 voltage-gated potassium channel-encoding gene KCNA1 is linked to human autosomal dominant hypomagnesemia. J Clin Invest. 2009;119(4):936–42.
Malicdan MCV, Vilboux T, Ben-Zeev B, Guo J, Eliyahu A, Pode-Shakked B, Dori A, Kakani S, Chandrasekharappa SC, Ferreira CR, Shelestovich N, Marek-Yagel D, Pri-Chen H, Blatt I, Niederhuber JE, He L, Toro C, Taylor RW, Deeken J, Yardeni T, Wallace DC, Gahl WA, Anikster Y. A novel inborn error of the coenzyme Q10 biosynthesis pathway: cerebellar ataxia and static encephalomyopathy due to COQ5 C-methyltransferase deficiency. Hum Mutat. 2018;39(1):69–79.
Traschutz A, Schirinzi T, Laugwitz L, Murray NH, Bingman CA, Reich S, Kern J, Heinzmann A, Vasco G, Bertini E, Zanni G, Durr A, Magri S, Taroni F, Malandrini A, Baets J, de Jonghe P, de Ridder W, Bereau M, Demuth S, Ganos C, Basak AN, Hanagasi H, Kurul SH, Bender B, Schols L, Grasshoff U, Klopstock T, Horvath R, van de Warrenburg B, Burglen L, Rougeot C, Ewenczyk C, Koenig M, Santorelli FM, Anheim M, Munhoz RP, Haack T, Distelmaier F, Pagliarini DJ, Puccio H, Synofzik M. Clinico-genetic, imaging and molecular delineation of COQ8A-ataxia: a multicenter study of 59 patients. Ann Neurol. 2020;88(2):251–63.
Paprocka J, Jezela-Stanek A, Tylki-Szymanska A, Grunewald S. Congenital disorders of glycosylation from a neurological perspective. Brain Sci. 2021;11(1):88.
Reily C, Stewart TJ, Renfrow MB, Novak J. Glycosylation in health and disease. Nat Rev Nephrol. 2019;15(6):346–66.
Schiff M, Roda C, Monin ML, Arion A, Barth M, Bednarek N, Bidet M, Bloch C, Boddaert N, Borgel D, Brassier A, Brice A, Bruneel A, Buissonniere R, Chabrol B, Chevalier MC, Cormier-Daire V, De Barace C, De Maistre E, De Saint-Martin A, Dorison N, Drouin-Garraud V, Dupre T, Echenne B, Edery P, Feillet F, Fontan I, Francannet C, Labarthe F, Gitiaux C, Heron D, Hully M, Lamoureux S, Martin-Coignard D, Mignot C, Morin G, Pascreau T, Pincemaille O, Polak M, Roubertie A, Thauvin-Robinet C, Toutain A, Viot G, Vuillaumier-Barrot S, Seta N, De Lonlay P. Clinical, laboratory and molecular findings and long-term follow-up data in 96 French patients with PMM2-CDG (phosphomannomutase 2-congenital disorder of glycosylation) and review of the literature. J Med Genet. 2017;54(12):843–51.
Medina-Cano D, Ucuncu E, Nguyen LS, Nicouleau M, Lipecka J, Bizot JC, Thiel C, Foulquier F, Lefort N, Faivre-Sarrailh C, Colleaux L, Guerrera IC, Cantagrel V. High N-glycan multiplicity is critical for neuronal adhesion and sensitizes the developing cerebellum to N-glycosylation defect. Elife. 2018;7:e38309.
Zschocke J. Disorders of the biosynthesis and breakdown of complex molecules. In: Hoffmann G, Zschocke J, Nyhan W, editors. Inherited metabolic diseases. 2016. p 9–12.
Bible E, Gupta P, Hofmann SL, Cooper JD. Regional and cellular neuropathology in the palmitoyl protein thioesterase-1 null mutant mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol Dis. 2004;16(2):346–59.
Macauley SL, Wozniak DF, Kielar C, Tan Y, Cooper JD, Sands MS. Cerebellar pathology and motor deficits in the palmitoyl protein thioesterase 1-deficient mouse. Exp Neurol. 2009;217(1):124–35.
Shacka JJ. Mouse models of neuronal ceroid lipofuscinoses: useful pre-clinical tools to delineate disease pathophysiology and validate therapeutics. Brain Res Bull. 2012;88(1):43–57.
Cao Y, Staropoli JF, Biswas S, Espinola JA, MacDonald ME, Lee JM, Cotman SL. Distinct early molecular responses to mutations causing vLINCL and JNCL presage ATP synthase subunit C accumulation in cerebellar cells. PLoS One. 2011;6(2):e17118.
Brenneman DE, Pearce DA, Kovacs A, DeFrees S. Pharmacological effects on ceroid lipofuscin and neuronal structure in Cln3 (Δex7/8) mouse brain cultures. J Mol Neurosci. 2017;63(1):100–14.
Jana M, Dutta D, Poddar J, Pahan K. Activation of PPARalpha exhibits therapeutic efficacy in a mouse model of juvenile neuronal ceroid lipofuscinosis. J Neurosci. 2023;43(10):1814–29.
De Munter S, Verheijden S, Regal L, Baes M. Peroxisomal disorders: a review on cerebellar pathologies. Brain Pathol. 2015;25(6):663–78.
Faust PL. Abnormal cerebellar histogenesis in PEX2 Zellweger mice reflects multiple neuronal defects induced by peroxisome deficiency. J Comp Neurol. 2003;461(3):394–413.
Evrard P, Caviness VS Jr, Prats-Vinas J, Lyon G. The mechanism of arrest of neuronal migration in the Zellweger malformation: an hypothesis bases upon cytoarchitectonic analysis. Acta Neuropathol. 1978;41(2):109–17.
Krysko O, Hulshagen L, Janssen A, Schutz G, Klein R, De Bruycker M, Espeel M, Gressens P, Baes M. Neocortical and cerebellar developmental abnormalities in conditions of selective elimination of peroxisomes from brain or from liver. J Neurosci Res. 2007;85(1):58–72.
Muller CC, Nguyen TH, Ahlemeyer B, Meshram M, Santrampurwala N, Cao S, Sharp P, Fietz PB, Baumgart-Vogt E, Crane DI. PEX13 deficiency in mouse brain as a model of Zellweger syndrome: abnormal cerebellum formation, reactive gliosis and oxidative stress. Dis Model Mech. 2011;4(1):104–19.
Uittenbogaard M, Chiaramello A. Mitochondrial biogenesis: a therapeutic target for neurodevelopmental disorders and neurodegenerative diseases. Curr Pharm Des. 2014;20(35):5574–93.
Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130(3):548–62.
Scaglia F, Wong LJ, Vladutiu GD, Hunter JV. Predominant cerebellar volume loss as a neuroradiologic feature of pediatric respiratory chain defects. AJNR Am J Neuroradiol. 2005;26(7):1675–80.
Lax NZ, Gorman GS, Turnbull DM. Review: Central nervous system involvement in mitochondrial disease. Neuropathol Appl Neurobiol. 2017;43(2):102–18.
Lax NZ, Hepplewhite PD, Reeve AK, Nesbitt V, McFarland R, Jaros E, Taylor RW, Turnbull DM. Cerebellar ataxia in patients with mitochondrial DNA disease: a molecular clinicopathological study. J Neuropathol Exp Neurol. 2012;71(2):148–61.
Mori O, Yamazaki M, Ohaki Y, Arai Y, Oguro T, Shimizu H, Asano G. Mitochondrial encephalomyopathy with lactic acidosis and stroke like episodes (MELAS) with prominent degeneration of the intestinal wall and cactus-like cerebellar pathology. Acta Neuropathol. 2000;100(6):712–7.
Sparaco M, Bonilla E, DiMauro S, Powers JM. Neuropathology of mitochondrial encephalomyopathies due to mitochondrial DNA defects. J Neuropathol Exp Neurol. 1993;52(1):1–10.
Hakonen AH, Goffart S, Marjavaara S, Paetau A, Cooper H, Mattila K, Lampinen M, Sajantila A, Lonnqvist T, Spelbrink JN, Suomalainen A. Infantile-onset spinocerebellar ataxia and mitochondrial recessive ataxia syndrome are associated with neuronal complex I defect and mtDNA depletion. Hum Mol Genet. 2008;17(23):3822–35.
Aldahmesh MA, Mohamed JY, Alkuraya HS, Verma IC, Puri RD, Alaiya AA, Rizzo WB, Alkuraya FS. Recessive mutations in ELOVL4 cause ichthyosis, intellectual disability, and spastic quadriplegia. Am J Hum Genet. 2011;89(6):745–50.
Gibberd FB, Billimoria JD, Goldman JM, Clemens ME, Evans R, Whitelaw MN, Retsas S, Sherratt RM. Heredopathia atactica polyneuritiformis: Refsum’s disease. Acta Neurol Scand. 1985;72(1):1–17.
Ferdinandusse S, Zomer AW, Komen JC, van den Brink CE, Thanos M, Hamers FP, Wanders RJ, van der Saag PT, Poll-The BT, Brites P. Ataxia with loss of Purkinje cells in a mouse model for Refsum disease. Proc Natl Acad Sci U S A. 2008;105(46):17712–7.
Ronicke S, Kruska N, Kahlert S, Reiser G. The influence of the branched-chain fatty acids pristanic acid and Refsum disease-associated phytanic acid on mitochondrial functions and calcium regulation of hippocampal neurons, astrocytes, and oligodendrocytes. Neurobiol Dis. 2009;36(2):401–10.
Schonfeld P, Kahlert S, Reiser G. In brain mitochondria the branched-chain fatty acid phytanic acid impairs energy transduction and sensitizes for permeability transition. Biochem J. 2004;383(Pt 1):121–8.
Patay Z. The cerebellum in amino and organic acidurias. Neuroradiol J. 2007;20(4):439–48.
Wajner M, Latini A, Wyse AT, Dutra-Filho CS. The role of oxidative damage in the neuropathology of organic acidurias: insights from animal studies. J Inherit Metab Dis. 2004;27(4):427–48.
Choi CG, Yoo HW. Localized proton MR spectroscopy in infants with urea cycle defect. AJNR Am J Neuroradiol. 2001;22(5):834–7.
Harding BN, Leonard JV, Erdohazi M. Ornithine carbamoyl transferase deficiency: a neuropathological study. Eur J Pediatr. 1984;141(4):215–20.
Helman G, Pacheco-Colon I, Gropman AL. The urea cycle disorders. Semin Neurol. 2014;34(3):341–9.
Madsen E, Gitlin JD. Copper and iron disorders of the brain. Annu Rev Neurosci. 2007;30:317–37.
Miyajima H. Aceruloplasminemia, an iron metabolic disorder. Neuropathology. 2003;23(4):345–50.
Mulholland PJ. Susceptibility of the cerebellum to thiamine deficiency. Cerebellum. 2006;5(1):55–63.
Phillips SC, Harper CG, Kril J. A quantitative histological study of the cerebellar vermis in alcoholic patients. Brain. 1987;110(Pt 2):301–14.
Bekri S, Kispal G, Lange H, Fitzsimons E, Tolmie J, Lill R, Bishop DF. Human ABC7 transporter: gene structure and mutation causing X-linked sideroblastic anemia with ataxia with disruption of cytosolic iron-sulfur protein maturation. Blood. 2000;96(9):3256–64.
Gonzalez-Cabo P, Palau F. Mitochondrial pathophysiology in Friedreich’s ataxia. J Neurochem. 2013;126(Suppl 1):53–64.
Koeppen AH. Friedreich’s ataxia: pathology, pathogenesis, and molecular genetics. J Neurol Sci. 2011;303(1–2):1–12.
Kuseyri Hubschmann O, Mohr A, Friedman J, Manti F, Horvath G, Cortes-Saladelafont E, Mercimek-Andrews S, Yildiz Y, Pons R, Kulhanek J, Oppeboen M, Koht JA, Podzamczer-Valls I, Domingo-Jimenez R, Ibanez S, Alcoverro-Fortuny O, Gomez-Alemany T, de Castro P, Alfonsi C, Zafeiriou DI, Lopez-Laso E, Guder P, Santer R, Honzik T, Hoffmann GF, Garbade SF, Sivri HS, Leuzzi V, Jeltsch K, Garcia-Cazorla A, Opladen T, D. International Working Group on Neurotransmitter Related and I. Harting. Brain MR patterns in inherited disorders of monoamine neurotransmitters: an analysis of 70 patients. J Inherit Metab Dis. 2021;44(4):1070–82.
Lim YT, Mankad K, Kinali M, Tan AP. Neuroimaging spectrum of inherited neurotransmitter disorders. Neuropediatrics. 2020;51(1):6–21.
Pearl PL, Capp PK, Novotny EJ, Gibson KM. Inherited disorders of neurotransmitters in children and adults. Clin Biochem. 2005;38(12):1051–8.
Ma S, Sun R, Jiang B, Gao J, Deng W, Liu P, He R, Cui J, Ji M, Yi W, Yang P, Wu X, Xiong Y, Qiu Z, Ye D, Guan KL. L2hgdh deficiency accumulates l-2-hydroxyglutarate with progressive leukoencephalopathy and neurodegeneration. Mol Cell Biol. 2017;37(8):e00492–16.
Funding
This work was supported by NIH grants 5K08NS110877-04 (IMV), Child Neurology Foundation (IMV), and Basil O’Connol Young Investigator Award (IMV).
Author information
Authors and Affiliations
Contributions
MGR and IMV conceived the project, created and curated the database, analyzed and interpreted the data, and wrote the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable. All data used in this study was obtained from public databases. No patient or family information has been used.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12311_2023_1641_MOESM1_ESM.pdf
Supplementary figure 1. Structural cerebellar phenotypes. Distributions of subgroups of IEM within each structural phenotype of the cerebellum. (PDF 1416 KB)
12311_2023_1641_MOESM2_ESM.pdf
Supplementary figure 2. Distribution of subgroups of disorders of Complex Molecule and Organelle Metabolism within the developmental cerebellar phenotype. Subgroups were distributed within the cerebellum and cerebellum+ groups. Specific structural developmental defects of the cerebellum within each subgroup were included. Given the prevalence of subgroups within the cerebellum+ group, relative frequencies of brain regions involved were shown as a heatmap panel. (PDF 576 KB)
12311_2023_1641_MOESM3_ESM.pdf
Supplementary figure 3. Distribution of subgroups of disorders of Intermediary Metabolism Energy and Complex Molecule and Organelle Metabolism within the degenerative cerebellar phenotype. Subgroups were distributed with the cerebellum and cerebellum+ groups. Relative frequencies of brain regions involved in the cerebellum + are illustrated in the heatmap. (PDF 1173 KB)
12311_2023_1641_MOESM4_ESM.pdf
Supplementary figure 4. Distribution of subgroups of disorders of Complex Molecule and Organelle Metabolism and Intermediary Metabolism Energy within the developmental and degenerative cerebellar phenotypes. Subgroups were distributed with the cerebellum and cerebellum+ groups. Specific structural developmental defects of the cerebellum within each subgroup is shown. Relative frequencies of brain regions involved are shown for the cerebellum+ group in the heatmap. (PDF 593 KB)
12311_2023_1641_MOESM5_ESM.pdf
Supplementary figure 5. Distribution of groups of IEM within the cerebellum and cerebellum+ group in other cerebellar phenotypes. Within the cerebellum and cerebellum+ groups, the presence or absence of cerebellar motor deficits, atrophy, and additional brain lesions are indicated. (PDF 18910 KB)
12311_2023_1641_MOESM6_ESM.pdf
Supplementary figure 6. Distribution of biochemical changes of other IEM groups within each cerebellar phenotype. (PDF 403 KB)
12311_2023_1641_MOESM7_ESM.pdf
Supplementary figure 7. Distribution of cerebellar structural lesions within each IEM group presenting with ataxia. (PDF 127 KB)
12311_2023_1641_MOESM8_ESM.pdf
Supplementary figure 8. Distribution of cerebellar deficits in disorders of Intermediary Metabolism Energy. (PDF 2966 KB)
12311_2023_1641_MOESM9_ESM.pdf
Supplementary figure 9. Relative frequencies of subgroups, cerebellar phenotypes, brain regions involved and type of lesions, and associated clinical deficits in other IEM groups. (PDF 492 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gonzalez-Rodriguez, M., Marin-Valencia, I. Metabolic Determinants of Cerebellar Circuit Formation and Maintenance. Cerebellum (2023). https://doi.org/10.1007/s12311-023-01641-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s12311-023-01641-2