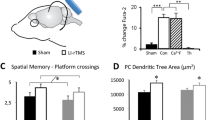
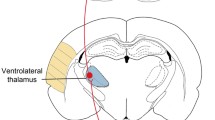
Abstract
Electromagnetic brain stimulation is a promising treatment in neurology and psychiatry. However, clinical outcomes are variable and underlying mechanisms remain ill-defined, impeding the development of new effective stimulation protocols. There is increasing application of repetitive transcranial magnetic stimulation (rTMS) to the cerebellum to induce forebrain plasticity through its long-distance cerebello-cerebral circuits. To better understand what magnetic stimulation does within the cerebellum, we have developed tools to generate defined low-intensity (LI) magnetic fields and deliver them in vivo, in 3D organotypic culture and in primary cultures, over a range of stimulation parameters. Here we show that low-intensity rTMS (LI-rTMS) to the cerebellum induces axon growth and synapse formation providing olivocerebellar reinnervation. This repair depends on stimulation pattern, with complex biomimetic patterns being most effective, and this requires the presence of a cellular magnetoreceptor, cryptochrome. To explain these reparative changes, we found that repair-promoting LI-rTMS patterns, but not ineffective ones, increased c-fos expression in Purkinje neurons, consistent with the production of reactive oxygen species by activated cryptochrome. Rather than activating neurons via induced electric currents, we propose that weak magnetic fields act through cryptochrome, activating intracellular signals that induce climbing fibre-Purkinje cell reinnervation. This information opens new routes to optimize cerebellar magnetic stimulation and its potential role as an effective treatment for neurological diseases.


Similar content being viewed by others
References
Pell GS, Roth Y, Zangen A. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: influence of timing and geometrical parameters and underlying mechanisms. Prog Neurobiol. 2011;93:59–98.
Grimaldi G, Argyropoulos GP, Boehringer A, Celnik P, Edwards MJ, Ferrucci R, Galea JM, Groiss SJ, Hiraoka K, Kassavetis P, et al. Non-invasive cerebellar stimulation–a consensus paper. Cerebellum. 2014;13:121–38.
Benussi A, Pascual-Leone A, Borroni B. Non-invasive cerebellar stimulation in neurodegenerative ataxia: a literature review. Int J Mol Sci. 2020;21:E1948.
Hatten ME. Adding cognitive connections to the cerebellum. Science. 2020;370:1411–2.
Rodger J, Mo C, Wilks T, Dunlop SA, Sherrard RM. Transcranial pulsed magnetic field stimulation facilitates reorganization of abnormal neural circuits and corrects behavioral deficits without disrupting normal connectivity. FASEB J. 2012;26:1593–606.
Grehl S, Viola HM, Fuller-Carter PI, Carter KW, Dunlop SA, Hool LC, Sherrard RM, Rodger J. Cellular and molecular changes to cortical neurons following low intensity repetitive magnetic stimulation at different frequencies. Brain Stimul. 2015;8:114–23.
Morellini N, Grehl S, Tang A, Rodger J, Mariani J, Lohof AM, Sherrard RM. What Does Low-Intensity rTMS Do to the Cerebellum? Cerebellum 2015;14:23–6. Available at: https://doi.org/10.1007/s12311-014-0617-9.
Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci. 2004;24:3379–85.
Welsh JP, Lang EJ, Suglhara I, Llinás R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374:453–7.
Dixon KJ, Sherrard RM. Brain-derived neurotrophic factor induces post-lesion transcommissural growth of olivary axons that develop normal climbing fibers on mature Purkinje cells. Exp Neurol. 2006;202:44–56.
Willson ML, McElnea C, Mariani J, Lohof AM, Sherrard RM. BDNF increases homotypic olivocerebellar reinnervation and associated fine motor and cognitive skill. Brain. 2008;131:1099–112.
Dufor T, Grehl S, Tang AD, Doulazmi M, Traoré M, Debray N et al. Neural circuit repair by low-intensity magnetic stimulation requires cellular magnetoreceptors and specific stimulation patterns. Sci Adv. 2019;5:eaav9847.
Makowiecki K, Harvey AR, Sherrard RM, Rodger J. Low-intensity repetitive transcranial magnetic stimulation improves abnormal visual cortical circuit topography and upregulates BDNF in mice. J Neurosci. 2014;34:10780–92.
Letellier M, Bailly Y, Demais V, Sherrard RM, Mariani J, Lohof AM. Reinnervation of late postnatal Purkinje cells by climbing fibers: Neosynaptogenesis without transient multi-innervation. J Neurosci. 2007;27:5373–83.
Chédotal A, Bloch-Gallego E, Sotelo C. The embryonic cerebellum contains topographic cues that guide developing inferior olivary axons. Development. 1997;124:861–70.
Letellier M, Wehrlé R, Mariani J, Lohof AM. Synapse elimination in olivo-cerebellar explants occurs during a critical period and leaves an indelible trace in Purkinje cells. Proc Natl Acad Sci USA. 2009;106:14102–7.
Grehl S, Martina D, Goyenvalle C, Deng Z-D, Rodger J, Sherrard RM. In vitro magnetic stimulation: a simple stimulation device to deliver defined low intensity electromagnetic fields. Front Neural Circuits. 2016;10:85.
Santulli G, Nakashima R, Yuan Q, Marks AR. Intracellular calcium release channels: an update. J Physiol. 2017;595:3041–51.
Görlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol. 2015;6:260–71.
Consentino L, Lambert S, Martino C, Jourdan N, Bouchet P-E, Witczak J, Castello P, El-Esawi M, Corbineau F, d’Harlingue A, et al. Blue-light dependent reactive oxygen species formation by Arabidopsis cryptochrome may define a novel evolutionarily conserved signaling mechanism. New Phytol. 2015;206:1450–62.
Müller P, Ahmad M. Light-activated cryptochrome reacts with molecular oxygen to form a flavin–superoxide radical pair consistent with magnetoreception. J Biol Chem. 2011;286:21033–40.
van Wilderen LJGW, Silkstone G, Mason M, van Thor JJ, Wilson MT. Kinetic studies on the oxidation of semiquinone and hydroquinone forms of Arabidopsis cryptochrome by molecular oxygen. FEBS Open Bio. 2015;5:885–92.
Chaves I, Pokorny R, Byrdin M, Hoang N, Ritz T, Brettel K, Essen L-O, van der Horst GTJ, Batschauer A, Ahmad M. The cryptochromes: blue light photoreceptors in plants and animals. Annu Rev Plant Biol. 2011;62:335–64.
Wiltschko R, Wiltschko W. Sensing magnetic directions in birds: radical pair processes involving cryptochrome. Biosensors (Basel). 2014;4:221–42.
Foley LE, Gegear RJ, Reppert SM. Human cryptochrome exhibits light-dependent magnetosensitivity. Nat Commun. 2011;2:356.
Sherrard RM, Morellini N, Jourdan N, El-Esawi M, Arthaut L-D, Niessner C et al. Low-intensity electromagnetic fields induce human cryptochrome to modulate intracellular reactive oxygen species. PLoS Biol. 2018;1:e2006229.
van der Horst GTJ, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker APM, van Leenen D, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–30.
Funding
The study was supported in part by the Institut de la Recherche sur la Moelle épinière et l’Encéphale and the Agence Nationale de la Recherche, project « BrainMag» (N° ANR-19-CE37-0021).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lohof, A.M., Dufor, T. & Sherrard, R.M. Neural Circuit Repair by Low-Intensity rTMS. Cerebellum 21, 750–754 (2022). https://doi.org/10.1007/s12311-021-01354-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-021-01354-4