Abstract
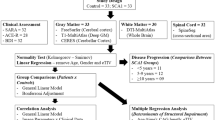
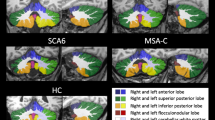
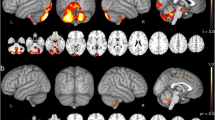
Spinocerebellar ataxias type 3 (SCA3) and type 10 (SCA10) are the most prevalent in southern Brazil. To analyze the relationships between volumetric MRI changes and clinical and genetic findings in SCA3 and SCA10 patients. All patients in the study had a confirmed genetic diagnosis. Demographic data, ataxia severity (SARA score), and the size of the expanded alleles were evaluated. Nineteen SCA3 and 18 SCA10 patients were selected and compared with a similar number of healthy controls. Patient and control groups underwent the same MRI protocol. The standard FreeSurfer pipeline was used for the morphometric data. Our results show more affected brain structures (volume reductions) in SCA3 patients than in SCA10 patients (15 vs. 5 structures). Volume reductions in brain structures were also greater in the former. The main areas with significant volumetric reductions in the former were the cerebellum, basal ganglia, brain stem, and diencephalon, whereas in the latter, significant volume reductions were observed in the cerebellum and pallidum. While SARA scores and disease duration were more correlated with volume reduction in SCA10, in SCA3, the expansion length (CAGn) correlated positively with cerebellar WM, thalamus, brain stem, and total GM volumes. There was no correlation between expansion length (ATTCTn) and neuroimaging findings in SCA10. Neuroimaging results differed significantly between SCA3 and SCA10 patients and were compatible with the differences in clinical presentation, disease progression, and molecular findings.

Similar content being viewed by others
References
Schols L, Bauer P, Schmidt T, Schulte T, Riess O. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol. 2004;3:291–4.
Bird T. Hereditary ataxia overview. Ncbi.nlm.nih.gov. 2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1138/
Teive HAG, Ashizawa T. Primary and secondary ataxias. Curr Opin Neurol. 2015;28:413–22.
Moro A, Munhoz RP, Moscovich M, Arruda WO, Raskin S, Silveira-Moriyama L, et al. Nonmotor symptoms in patients with spinocerebellar ataxia type 10. Cerebellum. 2017;6:1–7.
Ashizawa T. Spinocerebellar ataxia type 10. Handb Clin Neurol. 2012;103:507–19.
Teive HAG, Roa BB, Raskin S, Fang P, Arruda WO, Neto YC, et al. Clinical phenotype of Brazilian families with spinocerebellar ataxia 10. Neurology. 2004;63(8):1509–12.
Döhlinger S, Hauser T, Borkert J, Luft AR, Schulz JB. Magnetic resonance imaging in spinocerebellar ataxias. Cerebellum. 2008;7(2):204–14.
Kang J-S, Klein JC, Baudrexel S, Deichmann R, Nolte D, Hilker R. White matter damage is related to ataxia severity in SCA3. J Neurol. 2014;261(2):291–9.
Rasmussen A, Matsuura T, Ruano L, Yescas P, Ochoa A, Ashizawa T, et al. Clinical and genetic analysis of four Mexican families with spinocerebellar ataxia type 10. Ann Neurol. 2001;50(2):234–9.
Jacobi H, Hauser TK, Giunti P, Globas C, Bauer P, Schmitz-Hübsch T, et al. Spinocerebellar ataxia types 1, 2, 3 and 6: the clinical spectrum of ataxia and morphometric brain stem and cerebellar findings. Cerebellum. 2012;11(1):155–66.
Huang SR, Wu YT, Jao CW, Soong BW, Lirng JF, Wu HM, et al. CAG repeat length does not associate with the rate of cerebellar degeneration in spinocerebellar ataxia type 3. Neuroimage Clin. 2016;10(13):97–105.
Camargos ST, Marques-Jr W, Santos AC. Brain stem and cerebellum volumetric analysis of Machado Joseph disease patients. Arq Neuropsiquiatr. 2011;69(2b):292–6.
Stefanescu MR, Dohnalek M, Maderwald S, Thürling M, Minnerop M, Beck A, et al. Structural and functional MRI abnormalities of cerebellar cortex and nuclei in SCA3, SCA6 and Friedreich's ataxia. Brain. 2015;138(Pt 5):1182–97.
Klaes A, Reckziegel E, Franca MC, Rezende TJR, Vedolin LM, Jardim LB, et al. MR imaging in spinocerebellar ataxias: a systematic review. AJNR Am J Neuroradiol. 2016;37(8):1405–12.
Schmitz-Hübsch T, Tezenas du Montcel S, Baliko L, Boesch S, Bonato S, Fancellu R, et al. Reliability and validity of the International Cooperative Ataxia Rating Scale: a study in 156 spinocerebellar ataxia patients. Mov Disord. 2006;21(5):699–704.
Braga-Neto P, Godeiro-Junior C, Dutra LA, Pedroso JL, Barsottini OGP. Translation and validation into Brazilian version of the Scale of the Assessment and Rating of Ataxia (SARA). Arq Neuropsiquiatr. 2010;68:228–30.
Wessa P. Free Statistics Software, Office for Research Development and Education, version 1.2.1, 2019. Available from: https://www.wessa.net/
Fischl B. FreeSurfer. FreeSurfer Neuroimage. 2012 Aug 15;62(2):774–81. https://doi.org/10.1016/j.neuroimage.2012.01.021.
Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–18.
Fahl CN, Branco LMT, Bergo FPG, D’Abreu A, Lopes-Cendes I, França MC. Spinal cord damage in Machado-Joseph disease. Cerebellum. 2015;14(2):128–32.
Hernandez-Castillo CR, Diaz R, Vaca-Palomares I, Torres DL, Chirino A, Campos-Romo A, et al. Extensive cerebellar and thalamic degeneration in spinocerebellar ataxia type 10. Parkinsonism Relat Disord. 2019 Sep;66:182–8. https://doi.org/10.1016/j.parkreldis.2019.08.011.
McFarland KN, Liu J, Landrian I, Gao R, Sarkar PS, Raskin S, et al. Paradoxical effects of repeat interruptions on spinocerebellar ataxia type 10 expansions and repeat instability. Eur J Hum Genet. 2013;21(11):1272–6.
Rezende TJR, de Paiva JLR, Martinez ARM, Lopes-Cendes I, Pedroso JL, Barsottini OGP, et al. Structural signature of SCA3: from presymptomatic to late disease stages. Ann Neurol. 2018;84(3):401–8.
Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig: Barth; 1909.
Nascimento FA, Rodrigues VOR, Pelloso FC, Camargo CHF, Moro A, Raskin S, et al. Spinocerebellar ataxias in southern Brazil: genotypic and phenotypic evaluation of 213 families. Clin Neurol Neurosurg. 2019;184:105427.
Garrard P, Martin NH, Giunti P, Cipolotti L. Cognitive and social cognitive functioning in spinocerebellar ataxia: a preliminary characterization. J Neurol. 2008;255(3):398–405.
Bürk K, Globas C, Bösch S, Klockgether T, Zühlke C, Daum I, et al. Cognitive deficits in spinocerebellar ataxia type 1, 2, and 3. J Neurol. 2003;250(2):207–11.
Hoche F, Guell X, Vangel MG, Sherman JC, Schmahmann JD. The cerebellar cognitive affective/Schmahmann syndrome scale. Brain. 2017;141(1):248–70.
D’Abreu A, França MC Jr, Yasuda CL, Campos BA, Lopes-Cendes I, Cendes F. Neocortical atrophy in Machado-Joseph disease: a longitudinal neuroimaging study. J Neuroimaging. 2012;22(3):285–91.
Coutinho P, Guimarães A, Scaravilli F. The pathology of Machado-Joseph disease. Report of a possible homozygous case. Acta Neuropathol. 1982;58(1):48–54.
Lukas C, Schöls L, Bellenberg B, Rüb U, Przuntek H, Schmid G, et al. Dissociation of grey and white matter reduction in spinocerebellar ataxia type 3 and 6: a voxel-based morphometry study. Neurosci Lett. 2006;408:230–5.
Schulz JB, Borkert J, Wolf S, Schmitz-Hübsch T, Rakowicz M, Mariotti C, et al. Visualization, quantification and correlation of brain atrophy with clinical symptoms in spinocerebellar ataxia types 1, 3 and 6. Neuroimage. 2010;49(1):158–68.
Paulson HL, Das SS, Crino PB, Perez MK, Patel SC, Gotsdiner D, et al. Machado-Joseph disease gene product is a cytoplasmic protein widely expressed in brain. Ann Neurol. 1997;41(4):453–62.
Naito H, Takahashi T, Kamada M, Morino H, Yoshino H, Hattori N, et al. First report of a Japanese family with spinocerebellar ataxia type 10: the second report from Asia after a report from China. PLoS One. 2017;12(5):e0177955.
Wang K, McFarland KN, Liu J, Zeng D, Landrian I, Xia G, et al. Spinocerebellar ataxia type 10 in Chinese Han. Neurol Genet. 2015;1(3):e26.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Arruda, W.O., Meira, A.T., Ono, S.E. et al. Volumetric MRI Changes in Spinocerebellar Ataxia (SCA3 and SCA10) Patients. Cerebellum 19, 536–543 (2020). https://doi.org/10.1007/s12311-020-01137-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-020-01137-3