Abstract
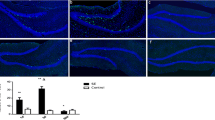
Status epilepticus (SE) promotes neuronal proliferation and differentiation in the adult and developing rodent hippocampus. However, the effect of SE on other neurogenic brain regions such as the cerebellum has been less explored. To determine whether SE induced by pentylentetrazole (PTZ-SE) and lithium-pilocarpine (Li-Pilo-SE) increases cell proliferation and neurogenesis in the developing rat cerebellum. SE was induced in 14-day-old (P14) Wistar rat pups (both sexes). One hour after SE and the following day rats were injected intraperitoneally with 5-bromo-2′-deoxyuridine (BrdU, 50 mg/kg). Seven days after SE, immunohistochemistry was performed to detect BrdU-positive (BrdU+) cells or BrdU/NeuN+ cells in the cerebellar vermis. SE induced by PTZ or Li-Pilo statistically significant increased the number of cerebellar BrdU+ cells when compared with the control group (58% and 40%, respectively); maximal cell proliferation occurred in lobules II, III, VIb, VIc, VIII, IXa, and IXb of PTZ-SE group and II, V, VIc, VII, and X of Li-Pilo-SE group. An increased number of BrdU/NeuN+ cells was detected in lobules V (17 ± 1.9), VIc (25.8 ± 2.7), and VII (26.2 ± 3.4) after Li-Pilo-SE compared to their control group (9.8 ± 1.7, 12.8 ± 2.8, and 11 ± 1.7, respectively), while the number of BrdU/NeuN+ cells remained the same after PTZ-induced SE or control conditions. SE induced in the developing rat by different experimental models increases cell proliferation in the granular layer of the cerebellar vermis, but only SE of limbic seizures increases neurogenesis in specific cerebellar lobes.



Similar content being viewed by others
References
Mohapel P, Ekdahl CT, Lindvall O. Status epilepticus severity influences the long-term outcome of neurogenesis in the adult dentate gyrus. Neurobiol Dis. 2004;15(2):196–205.
Cherian A, Thomas SV. Status epilepticus. Ann Indian Acad Neurol. 2009;12(3):140–53. https://doi.org/10.4103/0972-2327.56312.
Carreira BP, Santos DF, Santos AI, Carvalho CM, Araújo IM. Nitric oxide regulates neurogenesis in the hippocampus following seizures. Oxidative Med Cell Longev. 2015;451512. https://doi.org/10.1155/2015/451512.
Jiruska P, Shtaya AB, Bodansky DM, Chang WC, Gray WP, Jefferys JG. Dentate gyrus progenitor cell proliferation after the onset of spontaneous seizures in the tetanus toxin model of temporal lobe epilepsy. Neurobiol Dis. 2013;54:492–8. https://doi.org/10.1016/j.nbd.2013.02.001.
Varodayan FP, Zhu XJ, Cui XN, Porter BE. Seizures increase cell proliferation in the dentate gyrus by shortening progenitor cell cycle length. Epilepsia. 2009;50(12):2638–47. https://doi.org/10.1111/j.1528-1167.2009.02244.x.
Sankar R, Shin D, Liu H, Katsumori H, Wasterlain CG. Granule cell neurogenesis after status epilepticus in the immature rat brain. Epilepsia. 2000;41(Suppl 6):S53–6.
Hung YW, Yang DI, Huang PY, Lee TS, Kuo TB, Yiu CH, et al. The duration of sustained convulsive seizures determines the pattern of hippocampal neurogenesis and the development of spontaneous epilepsy in rats. Epilepsy Res. 2012;98(2–3):206–15. https://doi.org/10.1016/j.eplepsyres.2011.09.015.
Yang F, Wang JC, Han JL, Zhao G, Jiang W. Different effects of mild and severe seizures on hippocampal neurogenesis in adult rats. Hippocampus. 2008;18(5):460–8. https://doi.org/10.1002/hipo.20409.
Parent JM, Jessberger S, Gage FH, Gong C. Is neurogenesis reparative after status epilepticus? Epilepsia. 2007;48(s8):69–71.
Bengzon J, Kokaia Z, Elmér E, Nanobashvili A, Kokaia M, Lindvall O. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci U S A. 1997;94(19):10432–7.
Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78(3–5):272–303.
Voogd J, Glickstein M. The anatomy of the cerebellum. Trends Neurosci. 1998;21(9):370–5.
Cooper IS, Amin I, Riklan M, Waltz JM, Poon TP. Chronic cerebellar stimulation in epilepsy. Clinical and anatomical studies. Arch Neurol. 1976;33(8):559–70.
Cooper IS, Crighel E, Amin I. Clinical and physiological effects of stimulation of the paleocerebellum in humans. J Am Geriatr Soc. 1973;21(1):40–3.
Davis R, Emmonds SE. Cerebellar stimulation for seizure control: 17-year study. Stereotact Funct Neurosurg. 1992;58(1–4):200–8.
Gartside IB. The effects of cerebellectomy on a penicillin epileptogenic focus in the cerebral cortex of the rat. Electroencephalogr Clin Neurophysiol. 1978;44(3):373–9.
Paz C, Ferna A. Amygdala kindling in totally cerebellectomized cats. Exp Neurol. 1985;88(2):418–24.
Rubio C, Custodio V, González E, Retana-Márquez S, López M, Paz C. Effects of kainic acid lesions of the cerebellar interpositus and dentate nuclei on amygdaloid kindling in rats. Brain Res Bull. 2011;85(1–2):64–7. https://doi.org/10.1016/j.brainresbull.2011.02.003.
Zhu JN, Wang JJ. The cerebellum in feeding control: possible function and mechanism. Cell Mol Neurobiol. 2008;28(4):469–78.
Altman J. Autoradiographic and histological studies of postnatal neurogenesis. III. Dating the time of production and onset of differentiation of cerebellar microneurons in rats. J Comp Neurol. 1969;136(3):269–93.
Das GD, Nornes HO. Neurogenesis in the cerebellum of the rat: an autoradiographic study. Z Anat Entwicklungsgesch. 1972;138(2):155–65.
Bandeira F, Lent R, Herculano-Houzel S. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc Natl Acad Sci U S A. 2009;106(33):14108–13. https://doi.org/10.1073/pnas.0804650106.
Lomoio S, Necchi D, Mares V, Scherini E. A single episode of neonatal seizures alters the cerebellum of immature rats. Epilepsy Res. 2011;93(1):17–24. https://doi.org/10.1016/j.eplepsyres.2010.10.013.
Boop S, Wheless J, Van Poppel K, Mc Gregor A, Boop FA. Cerebellar seizures. Report of 2 cases. J Neurosurg Pediatr. 2013;12(3):288–92. https://doi.org/10.3171/2013.5.PEDS1394.
Kros L, Eelkman Rooda OH, Spanke JK, Alva P, van Dongen MN, Karapatis A, et al. Cerebellar output controls generalized spike-and-wave discharge occurrence. Ann Neurol. 2015;77(6):1027–49. https://doi.org/10.1002/ana.24399.
Velazco-Cercas E, Puig-Lagunes ÁA, Zamora-Bello I, Beltrán-Parrazal L, Morgado-Valle C, López-Meraz ML. Eneurobiología. 2017;8(17):220517.
Sankar R, Shin DH, Liu H, Mazarati A, Pereira de Vasconselos A, Wasterlain CG. Patterns of status epilepticus-induceed neuronal injury during development and longterm consequences. J Neurosci. 1998;18(20):8382–93.
Lopez-Meraz ML, Niquet J, Wasterlain CG. Distinct caspase pathways mediate necrosis and apoptosis in subpopulations of hippocampal neurons after status epilepticus. Epilepsia. 2010;51(Suppl 3):56–60. https://doi.org/10.1111/j.1528-1167.2010.02611.x.
Cameron HA, Mckay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435(4):406–17.
Moshé SL, Albala BJ, Ackermann RF, Engel J. Increased seizure susceptibility of the immature brain. Brain Res. 1983;283:81–5.
Shinnar S, Pellock JM, Moshé SL, Maytal J, O’Dell C, Driscoll SM, et al. In whom does status epilepticus occur: age-related differences in children. Epilepsia. 1997;38:907–14.
Haut SR, Velísková J, Moshé SL. Susceptibility of immature and adult brains to seizure effects. Lancet Neurol. 2004;3:608–17.
DeLorenzo RJ, Hauser WA, Towne AR, Boggs JG, Pellock JM, Penberthy L, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46:1029–35.
DeLorenzo RJ, Pellock JM, Towne AR, Boggs JG. Epidemiology of status epilepticus. J Clin Neurophysiol. 1995;12:316–25.
Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med. 1998;338:970–6.
Pohl M, Mares P. Effects of flunarizine on metrazol-induced seizures in developing rats. Epilepsy Res. 1987;1(5):302–5.
López-Meraz ML, Medel-Matus JS, Morgado-Valle C, Beltrán-Parrazal L, Pérez-Estudillo C, Manzo J. Effect of lithium-pilocarpine-induced status epilepticus on ultrasonic vocalizations in the infant rat pup. Epilepsy Behav. 2014;31:263–6. https://doi.org/10.1016/j.yebeh.2013.10.006.
Haas KZ, Sperber EF, Moshé SL. Kindling in developing animals: expression of severe seizures and enhanced development of bilateral foci. Brain Res Dev Brain Res. 1990;56(2):275–80.
Suchomelova L, Lopez-Meraz ML, Niquet J, Kubova H, Wasterlain CG. Hyperthermia aggravates status epilepticus-induced epileptogenesis and neuronal loss in immature rats. Neuroscience. 2015;305:209–24. https://doi.org/10.1016/j.neuroscience.2015.08.006.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–82. https://doi.org/10.1038/nmeth.2019.
Parent JM, Kron MM. Neurogenesis and epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies [Internet]. 4th edition. Bethesda (MD): National Center for Biotechnology Information (US); 2012.
Carletti B, Rossi F. Neurogenesis in the cerebellum. Neuroscientist. 2008 Feb;14(1):91–100.
Marzban H, Del Bigio MR, Alizadeh J, Ghavami S, Zachariah RM, Rastegar M. Cellular commitment in the developing cerebellum. Front Cell Neurosci. 2015;8:450. https://doi.org/10.3389/fncel.2014.00450.
Altman J, Bayer SA. Development of the cerebellar system: in relation to its evolution, structure and functions. Boca Raton: CRC Press; 1997.
Alder J, Cho NK, Hatten ME. Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron. 1996;17(3):389–99.
Hatten ME, Alder J, Zimmerman K, Heintz N. Genes involved in cerebellar cell specification and differentiation. Curr Opin Neurobiol. 1997;7(1):40–7.
Farrell JS, Nguyen QA, Soltesz I. Resolving the micro-macro disconnect to address core features of seizure networks. Neuron. 2019;101(6):1016–28. https://doi.org/10.1016/j.neuron.2019.01.043.
Hoffmann AF, Zhao Q, Holmes GL. Cognitive impairment following status epilepticus and recurrent seizures during early development: support for the “two-hit hypothesis”. Epilepsy Behav. 2004;5(6):873–7.
Paulin MG. The role of the Cerebellum in motor control and perception. Brain Behav Evol. 1993;41(1):39–50.
Galea JM, Vazquez A, Pasricha N, Orban de Xivry JJ, Celnik P. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex. 2011;21(8):1761–70.
Manzo J, Miquel M, Toledo R, Mayor-Mar JA, Garcia LI, Aranda-Abreu GE, et al. Fos expression at the cerebellum following non-contact arousal and mating behavior in male rats. Physiol Behav. 2008;93(1–2):357–63.
Anderson CM, Maas LC, Deb Frederick B, Bendor JT, Spencer TJ, Livni E, et al. Cerebellar vermis involvement in cocaine-related behaviors. Neuropsychopharmacology. 2006;31(6):1318–26.
Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17(10):3727–38.
Fujikawa DG. The temporal evolution of neuronal damage from pilocarpine-induced status epilepticus. Brain Res. 1996;725(1):11–22.
Cerebellum VJ. In: Paxinos G, editor. The Rat Nervous System: Elsevier; 2004. p. 133–90.
Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–44. https://doi.org/10.1016/j.cortex.2009.11.008.
Hagihara H, Hara M, Tsunekawa K, Nakagawa Y, Sawada M, Nakano K. Tonic–clonic seizures induce division of neuronal progenitor cells with concomitant changes in expression of neurotrophic factors in the brain of pilocarpine–treated mice. Brain Res Mol Brain Res. 2005;139(2):258–66.
Cameron HA, Hazel TG, McKay RD. Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol. 1998;36(2):287–306.
Danzer SC, He X, McNamara JO. Ontogeny of seizure-induced increases in BDNF immunoreactivity and TrkB receptor activation in rat hippocampus. Hippocampus. 2004;14(3):345–55.
Zucchini S, Barbieri M, Simonato M. Alterations in seizure susceptibility and in seizure-induced plasticity after pharmacologic and genetic manipulation of the fibroblast growth factor-2 system. Epilepsia. 2005;46(Suppl 5):52–8.
Dahmane N, Ruiz i Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126(14):3089–100.
Feng S, Ma S, Jia C, Su Y, Yang S, Zhou K, et al. Sonic hedgehog is a regulator of extracellular glutamate levels and epilepsy. EMBO Rep. 2016;17(5):682–94.
Fernández C, Tatard VM, Bertrand N, Dahmane N. Differential modulation of Sonic-hedgehog-induced cerebellar granule cell precursor proliferation by the IGF signaling network. Dev Neurosci. 2010;32(1):59–70.
Jiang G, Wang W, Cao Q, Gu J, Mi X, Wang K, et al. Insulin growth factor-1 (IGF-1) enhances hippocampal excitatory and seizure activity through IGF-1 receptor-mediated mechanisms in the epileptic brain. Clin Sci (Lond). 2015;129(12):1047–60. https://doi.org/10.1042/CS20150312.
Banerjee SB, Rajendran R, Dias BG, Ladiwala U, Tole S, Vaidya VA. Recruitment of the Sonic hedgehog signaling cascade in electroconvulsive seizure-mediated regulation of adult rat hippocampal neurogenesis. Eur J Neurosci. 2005;22(7):1570–80.
Koutsouraki ES, Anastasiades JJ, Baloyannis SJ. An immunohistochemical study of N-methyl-D-aspartate receptors in human cerebellum and hippocampus. American Journal of Medical Sciences and Medicine. 2013;1(2):28–30. https://doi.org/10.12691/ajmsm-1-2-3.
Wasterlain CG, Naylor DE, Liu H, Niquet J, Baldwin R. Trafficking of NMDA receptors during status epilepticus: therapeutic implications. Epilepsia. 2013;54(Suppl 6):78–80. https://doi.org/10.1111/epi.12285.
Wasterlain CG, Liu H, Mazarati AM, Baldwin RA, Shirasaka Y, Katsumori H, et al. Self-sustaining status epilepticus: a condition maintained by potentiation of glutamate receptors and by plastic changes in substance P and other peptide neuromodulators. Epilepsia. 2000;41(Suppl. 6):S134–43.
Yen W, Williamson J, Bertram EH, Kapur J. A comparison of three NMDA receptor antagonists in the treatment of prolonged status epilepticus. Epilepsy Res. 2004;59:43–50.
Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11(3):327–35.
Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca 2+−and stimulus duration–dependent switch for hippocampal gene expression. Cell. 1996;87(7):1203–14.
Hirsch E, Baram TZ, Snead OC 3rd. Ontogenic study of lithium–pilocarpine-induced status epilepticus in rats. Brain Res. 1992;583(1–2):120–6.
Ramanjaneyulu R, Ticku MK. Interactions of pentamethylenetetrazole and tetrazole analogues with the picrotoxinin site of the benzodiazepine- GABA receptorionophore complex. Eur J Pharmacol. 1984;98(3–4):337–45.
Turski L, Ikonomidou C, Turski WA, Bortolotto ZA, Cavalheiro EA. Cholinergic mechanisms and epileptogenesis: the seizures induced by pilocarpine: a novel. Experimental model of intractable epilepsy. Synapse. 1989;3(2):154–71.
Velísek L, Kubová H, Pohl M, Stanková L, Mares P, Schickerová R. Pentylenetetrazol-induced seizures in rats: an ontogenetic study. Naunyn Schmiedeberg's Arch Pharmacol. 1992;346(5):588–91.
Velísek L. Models of Chemically- Induced Acute Seizures. In: Pitkanen A, Schwartzkroin PA, Moshe SL, editors. Models of seizures and epilepsy: Elsevier Academic Press; 2006. p. 127–52.
Mareš P, Velišek L. N-methyl-D-aspartate (NMDA)-induced seizures in developing rats. Developmental brain research. Brain Res Dev Brain Res. 1992;65(2):185–9.
Kubová H, Rejchrtová J, Redkozubova O, Mareš P. Outcome of status epilepticus in immature rats varies according to the paraldehyde treatment. Epilepsia. 2005;46(Suppl 5):38–42.
Acknowledgments
This study was supported by CONACYT through the doctoral fellowship awarded to EVC (registration number 326059) and by Cuerpo Académico de Neurofisiología, Universidad Veracruzana (UV-CA-333).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Velazco-Cercas, E., Beltran-Parrazal, L., Morgado-Valle, C. et al. Status Epilepticus Increases Cell Proliferation and Neurogenesis in the Developing Rat Cerebellum. Cerebellum 19, 48–57 (2020). https://doi.org/10.1007/s12311-019-01078-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-019-01078-6