Abstract
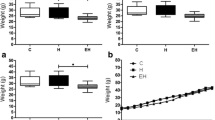
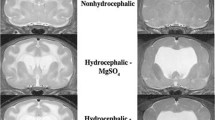
Hydrocephalus is a developmental disorder causing abnormally collected cerebrospinal fluid within the cerebral ventricles. It leads to bigger skulls and many dysfunctions related to the nervous system. Here, we addressed whether exogenous melatonin administration could reverse the clinical features of kaolin-induced hydrocephalus in infantile rats. A controlled double-blinded study was conducted in 2-week-old 45 Wistar albino rats, which were divided into three groups: Group A, the control group, received intracisternal sham injection with solely the needle insertion; group B, the hydrocephalus group, was treated with isotonic NaCl after kaolin injection; and group C, the hydrocephalus + melatonin group, was given i.p. exogenous melatonin at a dose of 0.5 mg/100 g body weight after kaolin injection. Histological and immunohistochemical analyses were performed after the induction of hydrocephalus and melatonin administration. Glial fibrillary acidic protein was stained by immunohistochemical method. TUNEL method was used to define and quantitate apoptosis in the cerebellar tissues. Statistical analysis was performed by nonparametric Kruskal–Wallis H test, and once significance was determined among means, post hoc pairwise comparisons were carried out using Mann–Whitney U test. We found that melatonin administration significantly ameliorated ratio of substantia grisea area/substantia alba area in the cerebellum of infantile rats. Histologically, there was a significant reduction in the number of cerebellar apoptotic cells after the hydrocephalus induced by kaolin (P < 0.05). Our results clearly revealed that the histopathological changes in the cerebellum were reversed by systemic melatonin administration in infantile rats with kaolin-induced hydrocephalus. Nevertheless, further studies are needed to suggest melatonin as a candidate protective drug in children with hydrocephalus.







Similar content being viewed by others
References
Okii N, Amano T, Seki T, Matsubayashi H, Mukai H, Ono Y, et al. Fragmentation of protein kinase N (PKN) in the hydrocephalic rat brain. Acta Histochem Cytochem. 2007;40(4):113–21.
Kondziella D, Lüdemann W, Brinker T, Sletvold O, Sonnewald U. Alterations in brain metabolism, CNS morphology and CSF Dynamics in adult rats with kaolin-induced hydrocephalus. Brain Res. 2002;927(1):35–41.
Kondziella D, Qu H, Lüdemann W, Brinker T, Sletvold O, Sonnewald U. Astrocyte metabolism is disturbed in the early development of experimental hydrocephalus. J Neurochem. 2003;85(1):274–81.
Ding Y, McAllister 2nd JP, Yao B, Yan N, Canady AI. Neuron tolerance during hydrocephalus. Neuroscience. 2001;106(4):659–67.
Fletcher JM, Landry SH, Bohan TP, Davidson KC, Brookshire BL, Lachar D, et al. Effects of intraventricular hemorrhage and hydrocephalus on the long-term neurobehavioral development of preterm very-low-birth weight infants. Dev Med Child Neurol. 1997;39(9):596–606.
Barkovich AJ. Pediatric neuroimaging. 3rd ed. Philadelphia: Williams and Wilkins; 2000. p. 253–81.
Redzic ZB, Segal MB. The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Adv Drug Deliv Rev. 2004;56(12):1695–716.
Turgut M, Erdogan S, Ergin K, Serter M. Melatonin ameliorates blood–brain barrier permeability, glutathione, and nitric oxide levels in the choroid plexus of the infantile rats with kaolin-induced hydrocephalus. Brain Res. 2007;1175:117–25.
Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7.
Hatta J, Hatta T, Moritake K, Otani H. Heavy water inhibiting the expression of transforming growth factor-beta1 and the development of kaolin-induced hydrocephalus in mice. J Neurosurg. 2006;104(4 Suppl):251–8.
Mashayekhi F, Salehi Z. Expression of nerve growth factor in cerebrospinal fluid of congenital hydrocephalic and normal children. Eur J Neurol. 2005;12(8):632–7.
Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology and neurobehavioral. Ann N Y Acad Sci. 2008;1142:266–309.
Del Bigio MR. Neuropathology and structural changes in hydrocephalus. Dev Disabil Res Rev. 2010;16:16–22.
Peach B. Arnold-Chiari malformation: anatomic features of 20 cases. Arch Neurol. 1965;12:613–21.
Pearce JM. Historical note. Arnold Chiari, or “Cruvilhier Cleland Chiari” malformation. J Neurol Neurosurg Psychiatry. 2000;68(1):13.
Bejjani GK. Definition of the adult Chiari malformation: a brief historical overview. Neurosurg Focus. 2001;11:E1.
Emery JL, Gadsdon DR. A quantitative study of the cell population of the cerebellum in children with myelomeningocele. Dev Med Child Neurol Suppl. 1975;35:20–5.
Boltshauser E. Cerebellum-small brain but large confusion: a review of selected cerebellar malformations and disruptions. Am J Med Genet A. 2004;126A:376–85.
Poretti A, Prayer D, Boltshauser E. Morphological spectrum of prenatal cerebellar disruptions. Eur J Paediatr Neurol. 2009;13:397–407.
Socci DJ, Bjugstad KB, Jones HC, Pattisapu JV, Arendash GW. Evidence that oxidative stress is associated with the pathophysiology of inherited hydrocephalus in the H-Tx rat model. Exp Neurol. 1999;155:109–17.
Botfield H, Gonzalez AM, Abdullah O, Skjolding AD, Berry M, McAllister 2nd JP, et al. Decorin prevents the development of juvenile communicating hydrocephalus. Brain. 2013;136(9):2842–58.
Cabuk B, Etus V, Bozkurt SU, Sav A, Ceylan S. Neuroprotective effect of memantine on hippocampal neurons in infantile rat hydrocephalus. Turk Neurosurg. 2011;21(3):352–8.
Catalão CH, Correa DA, Saito ST, Lopes LS. Camellia sinensis neuroprotective role in experimentally induced hydrocephalus in Wistar rats. Childs Nerv Syst. 2014;30(4):591–7.
Reiter R, Tang L, Garcia JJ, Muñoz-Hoyos A. Pharmacological actions of melatonin in oxygen radical pathophysiology. Life Sci. 1997;60(25):2255–71.
Uyanikgil Y, Turgut M, Ateş U, Baka M, Yurtseven ME. Beneficial effects of melatonin on morphological changes in postnatal cerebellar tissue owing to epileptiform activity during pregnancy in rats: light and immunohistochemical study. Brain Res Dev Brain Res. 2005;159(2):79–86.
Uyanikgil Y, Baka M, Ateş U, Turgut M, Yavaşoğlu A, Ulker S, et al. Neuroprotective effects of melatonin upon the offspring cerebellar cortex in the rat model of BCNU-induced cortical dysplasia. Brain Res. 2007;1160:134–44.
Balasubramaniam J, Del Bigio MR. Analysis of age-dependant alteration in the brain gene expression profile following induction of hydrocephalus in rats. Exp Neurol. 2002;173(1):105–13.
Hascalik S, Celik O, Karakas HM, Parlakpinar H, Firat AK, Ozsahin M. Protective role of melatonin in pinealectomized rat brains: in vivo magnetic resonance spectroscopic analysis. J Pineal Res. 2005;39(4):342–5.
Taysi S, Koc M, Büyükokuroğlu ME, Altinkaynak K, Sahin YN. Melatonin reduces lipid peroxidation and nitric oxide during irradiation-induced oxidative injury in the rat liver. J Pineal Res. 2003;34(3):173–7.
Wong CS, Jow GM, Kaizaki A, Fan LW, Tien LT. Melatonin ameliorates brain injury induced by systemic lipopolysaccharide in neonatal rats. Neuroscience. 2014;267:147–56.
Williams MT, Braun AA, Amos-Kroohs RM, McAllister 2nd JP, Lindquist DM, Mangano FT, et al. Kaolin-induced ventriculomegaly at weaning produces long-term learning, memory, and motor deficits in rats. Int J Dev Neurosci. 2014;35:7–15.
Crews L, Wyss-Coray T, Masliah E. Insights into the pathogenesis of hydrocephalus from transgenic and experimental animal models. Brain Pathol. 2004;14(3):312–6.
Air EL, Yuan W, Holland SK, Jones BV, Bierbrauer K, Altaye M, et al. Longitudinal comparison of pre- and postoperative diffusion tensor imaging parameters in young children with hydrocephalus. J Neurosurg Pediatr. 2010;5(4):385–91.
Chumas PD, Drake JM, Del Bigio MR, Da Silva M, Tuor UI. Anaerobic glycolysis preceding white-matter destruction in experimental neonatal hydrocephalus. J Neurosurg. 1994;80(3):491–501.
Del Bigio MR, Kanfer JN, Zhang YW. Myelination delay in the cerebral white matter of immature rats with kaolin-induced hydrocephalus is reversible. J Neuropathol Exp Neurol. 1997;56(9):1053–66.
Cinalli G, Maixner WJ, Sainte-Rose C, Silva MCD. Pathophysiology of hydrocephalus, eds Pediatric hydrocephalus. Milano: Springer; 2004. p. 65–76.
McAllister 2nd J. Pathophysiology of congenital and neonatal hydrocephalus. Semin Fetal Neonatal Med. 2012;17(5):285–94.
Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50(4):427–34.
Eskandari R, Harris CA, McAllister 2nd JP. Reactive astrocytosis in feline neonatal hydrocephalus: acute, chronic, and shunt-induced changes. Childs Nerv Syst. 2011;27(12):2067–76.
Hatten ME. Central nervous system neuronal migration. Annu Rev Neurosci. 1999;22:511–39.
Yamada K, Watanabe M. Cytodifferentiation of Bergmann glia and its relationship with Purkinje cells. Anat Sci Int. 2002;77(2):94–108.
Altman J, Bayer SA. The site of origin, mode of dispersal, and local proliferation of cerebellar glial cells. Development of the cerebellar system: in relation to its evolution, structure, and functions. New York: CRC Press; 1997. p. 252–64.
Hatten ME. The role of migration in central nervous system neuronal development. Curr Opin Neurobiol. 1993;3(1):38–44.
Rakic P. Principles of neural cell migration. Experientia. 1990;46(9):882–91.
Yachnis AT, Rorke LB. Cerebellar and brainstem development: an overview in relation to Joubert syndrome. J Child Neurol. 1999;14(9):570–3.
Kolb B, Sutherland RJ. Noradrenaline depletion blocks behavioral sparing and alters cortical morphogenesis after neonatal frontal cortex damage in rats. J Neurosci. 1992;12(6):2321–30.
Uyanikgil Y, Baka M, Yurtseven M, Turgut M. The effect of experimental epilepsy induced by penicillin administration during pregnancy on nestin expression in the immature rat cerebellum. A light, electron microscopic, and immunohistochemical study. Childs Nerv Syst. 2004;20(3):176–82.
Lekic T, Manaenko A, Rolland W, Virbel K, Hartman R, Tang J, et al. Neuroprotection by melatonin after germinal matrix hemorrhage in neonatal rats. Acta Neurochir Suppl. 2011;111:201–6.
Al-Ghoul WM, Herman MD, Dubocovich ML. Melatonin receptor subtype expression in human cerebellum. Neuroreport. 1998;9(18):4063–8.
Guerrero JM, Reiter RJ. A brief survey of pineal-immune system interrelationships. Endocr Res. 1992;18(2):91–113.
Leppert D, Wieser HG. Pregnancy, contraception and epilepsy. Nervenarzt. 1993;64(8):494–503.
Reiter RJ. Melatonin: lowering the high price of free radicals. News Physiol Sci. 2000;15:246–50.
Sandyk R. Possible role of pineal melatonin in the mechanisms of aging. Int J Neurosci. 1990;52(1–2):85–92.
Alonso-Alconada D, Álvarez A, Arteaga O, Martínez-Ibargüen A, Hilario E. Neuroprotective effect of melatonin: a novel therapy against perinatal hypoxia-ischemia. Int J Mol Sci. 2013;14(5):9379–95.
Bolduc ME, du Plessis AJ, Sullivan N, Guizard N, Zhang X, Robertson RL, et al. Regional cerebellar volumes predict functional outcome in children with cerebellar malformations. Cerebellum. 2012;11(2):531–42.
Watanabe M, Miyajima M, Nakajima M, Arai H, Ogino I, Nakamura S, et al. Expression analysis of high mobility group box-1 protein (HMGB-1) in the cerebral cortex, hippocampus, and cerebellum of the congenital hydrocephalus (H-Tx) rat. Acta Neurochir Suppl. 2012;113:91–6.
Watanabe M, Miyajima M, Ogino I, Nakajima M, Arai H. Cerebellar Purkinje cells exhibit increased expression of HMGB-1 and apoptosis in congenital hydrocephalic H-Tx rats. Neurosurgery. 2013;72(3):459–67.
Acknowledgments
Authors would like to thank Erdinç Yılmaz and Sevim Balkız for their excellent technical assistance and N. Ceren Sümer Turanlıgil for linguistic support. Also, we would like to express our great thanks to Professor Dr. Juan F. Martínez-Lage, Professor Dr. Abhaya V. Kulkarni, and Professor Dr. Daniel Cardinali for their critical reading of the manuscript and for their comments on an earlier version of the manuscript, and we sincerely thank the two “anonymous” reviewers for their insight full comments on our manucript. This study was presented in part at the 13th National Congress of Histology and Embryology Society, Cesme, Turkey, April 30-May 3, 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest either directly or indirectly related to this research.
Additional information
Yiğit Uyanıkgil and Mehmet Turgut contributed equally to this work.
Rights and permissions
About this article
Cite this article
Uyanıkgil, Y., Turgut, M. & Baka, M. Effects of Melatonin on the Cerebellum of Infant Rat Following Kaolin-Induced Hydrocephalus: a Histochemical and Immunohistochemical Study. Cerebellum 16, 142–150 (2017). https://doi.org/10.1007/s12311-016-0778-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-016-0778-9