Abstract
Epstein-Barr virus (EBV) typically infects B cells in infectious mononucleosis (IM), but a rare case shows EBV infection in T cells. Seven cases of lymphoproliferative disorder caused by EBV-positive cytotoxic T/natural killer (NK) cell proliferation in the lymph nodes, termed IM with transient EBV infection of T and NK cells (EBV + T/NK cells in IM), are reported here. The purpose of the study is to describe clinicopathological features of EBV + T/natural killer (NK) cells in IM of the lymph node. We retrospectively analysed seven cases of Chinese children and young people adults with EBV + T/NK cells in IM. We used morphological observation, immunohistochemical staining, EB virus in situ hybridisation detection, and analysis of T-cell receptor gene rearrangement. The patients were healthy prior to illness, experiencing sudden onset occurring in all the patients, with high fever as the first symptom, followed by lymphadenopathy and hepatosplenomegaly. Diagnosis occurred < 1.5 months of symptom onset. Most lymphocytes in lesions expressed CD3 and Granzyme B or TIA-1 and lacked CD5. CD56 was expressed in numerous cells in 5 of the 7 cases. EBV-encoded RNA (EBER) was detected in medium-to-large-sized cells (50–100 cells per cell/high-power field). T-cell receptor (TCR) gene rearrangement was seen in six cases, with monoclonal rearrangement in four cases. Treatment was conservative treatment but not chemotherapy. Four received anti-HLH therapy and others anti-inflammatory treatment. All patients survived with relapse after long-term clinical observation and follow-up. EBV + T/NK cells in IM can elicit malignant features that mimic T/NK-cell lymphoma pathologically and benign features mimicking IM clinically. These findings indicate that EBV + T/NK cells in IM could serve as valuable diagnosis. Additional clinical information, including age of onset (children and young people), nature of onset (sudden), disease course (short), symptoms (systemic), EBV infection status (acute), and lymph node involvement, is crucial for accurate diagnosis and prognostic evaluation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Epstein-Barr virus (EBV) is a member of the herpes virus family with double-stranded DNA and was discovered by Epstein in 1964 through isolation from Burkitt’s lymphoma [1]. Generally, EBV infection occurs early in life and is usually asymptomatic. However, if primary EBV infection is delayed until adolescence and young adulthood, it can result in infectious mononucleosis (IM). IM is a self-limiting lymphoid disease with a benign clinical course [2], characterised by most EBV-infected cells being B cells with few T and natural killer (NK) cells [3]. Additionally, EBV is linked to malignancies, such as classical Hodgkin’s lymphoma, Burkitt’s lymphoma, T-cell lymphoma, and NK cell lymphoma. Recently, EBV has been associated with chronic active EBV infection (CAEBV) and T/NK cell lymphoproliferative disease (LPDs) [4].
Systemic EBV-positive T-cell lymphoma in children has been defined by the World Health Organisation (WHO) [5] as developing shortly after primary or acute EBV infection in previously healthy children and young adults or in the CAEBV setting. Historically, this process has been described using various terms, including fulminant EBV + T-cell LPD in childhood, sporadic fatal IM, fulminant hemophagocytic syndrome in children in Taiwan, fatal EBV-associated hemophagocytic syndrome in Japan, and severe CAEBV infection. Although it is a systemic disease, it mostly involves the liver and spleen, followed by the lymph nodes, bone marrow (BM), skin, and lungs. Patients with this disease usually die within days to months of diagnosis [6]. Ohshima et al. [7] proposed a new nomenclature to classify the pathological categories of EBV + T/NK-LPD in 2008, consisting of categories A1, A2, A3, and B. Category B is described as a monomorphic LPD with clonality and a fulminant course. In most studies, it has been described as a life-threatening disease.
EBV typically infects B cells in the IM, although a rare case has shown EBV infection in T cells [8]. We report seven cases of lymphoproliferative disorder caused by EBV-positive cytotoxic T/NK cell proliferation in the lymph nodes. We designated this condition as IM with transient EBV infection of T and NK cells (EBV + T/NK cells in IM). Notably, EBV + T/NK cells in the IM are easily misdiagnosed as T/NK cell lymphoma because they share similar histological and immunohistochemical features, including a cytotoxic T/NK cell phenotype and EBV positivity. However, based on its clinical characteristics, that is, spontaneous regression within a short time, EBV + T/NK cells in the IM should be recognised as reactive.
Materials and methods
Case selection and morphologic review
Information on seven cases with acute EBV infections of T/NK cells was obtained retrospectively from the files of the Department of Pathology, Beijing Friendship Hospital, Capital Medical University (Lymphoma Diagnosis and Research Center, Institute of Beijing Clinical Medicine). These cases were received for consultation during the period of June 2013 to June 2018. The final follow-up was on September 25, 2021. The common features among these seven cases of EBV + T/NK cells in IM were (1) T/NK cell predominant lymphoproliferation in the lymph node, (2) EBV infection primarily of cytotoxic T/NK cells, (3) EBV-positive cells of > 50/high-power field (HPF), (4) no radiotherapy or chemotherapy, and (6) long-term follow-up for a.
Clinical information included sex, age, duration of disease, initial symptoms, laboratory test results of blood and Epstein-Barr virus (EBV) detection, imaging examination of the liver and spleen, and follow-up results. Morphological observations included (1) lymph node structural change and degree of change, (2) complexity of T/NK cell type and composition, (3) presence of necrosis, and (4) lymphocyte atypia.
Immunohistochemistry and EBER in situ hybridisation
Immunohistochemistry(IHC) staining was manually performed on formalin-fixed, paraffin-embedded (FFPE) tissues for immunophenotypic analysis. The MaxVision™ 2 kit (Cat. No. KIT-5910/5931) and monoclonal antibodies, against CD21, CD20, CD3, CD2, CD5, CD4, CD8, CD56, CD30, Granzyme B, TIA-1, PAX5, LMP1, and Ki-67, were provided by Maxin. Bio (provided by Maxin Bio, Fuzhou, CN, USA) and EBNA2 (provided by ABCOM) were used to detect all relevant antigens. Positive and negative controls were analysed according to the manufacturer’s instructions. The EBV Probe In Situ Hybridization Kit (Triplex International Biosciences (China) Co. Ltd., Fuzhou, China) was used to detect EBERs. Details of this procedure are described in our previous report [9]. The observers counted only definite EBV-positive cells, selecting fields with a high positivity rate. The total number of EBV-positive cells per high-power field (HPF) was recorded.
Double staining
The immunohistochemical and EBER in situ hybridisation dual staining was conducted using a Leica Bond-III autostainer (Leica, Melbourne, Australia). Sections, 2-μm thick, were initially stained for EBER using DAB staining (brown); then, for CD3 (CD3; Maxin) and CD20 (L26; Maxin) was performed, the red staining was visualised after application of amino-ethylcarbazole (Bond Polymer Refine Red Detection kit, Leica) as chromogen [9].
T-cell receptor gene clonality analysis
TCR gene rearrangement analysis was performed using the ‘Biomed-2’ primers (InVivoScribe Technologies, San Diego, CA, USA) [10]. DNA extracted from FFPE tissue samples utilised the TIANamp FFPE DNA Kit (DP331) (TIANGEN, Beijing, China). For the gene rearrangement assay, PCR reactions were conducted in a 25-μL volume containing 22.5 μL of master mix, 0.13 μL of AmpliTaq Gold DNA polymerase, and 100 ng of genomic DNA. The cycling profile comprised initial denaturation at 95 °C for 7 min, followed by 35 cycles of 95 °C for 45 s, 60 °C for 45 s, and 72 °C for 90 s; with a final extension at 72 °C for 10 min. Post-amplification, the PCR products were denatured at 94 °C for 5 min, followed by a quick chill to re-anneal the PCR products at 4 °C for at least 60 min and then electrophoresed on 6% polyacrylamide gels (Bio-Rad) in 1 × TBE buffer at 120 V for approximately 65 min. Gel was subsequently soaked for 20 min in 100 mL of 0.1 M NaCl solution containing 10 μL of 10 mg/mL Gel Red (Biotium, USA) and visualised under ultraviolet illumination for photography.
Results
Clinical features
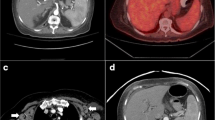
The clinical characteristics of the seven patients are summarised in Table 1. Five patients were male and two were female. The age of the patients ranged from 10 months to 19 years, with a median age of 5.0 years. All the patients were children and young adults. They were healthy prior to the illness. Sudden onset was observed in all patients, with high fever as the first symptom, followed by lymphadenopathy and hepatosplenomegaly based on computed tomography (CT) (Fig. 1A, case 5). The disease course at diagnosis ranged from 1 to 1.5 months. Patients (patients 3 and 4) presented with pleural effusion and severe pneumonia. Laboratory examinations revealed reduced white blood cell counts at different levels in all the patients. Thrombocytopenia was observed in 4/7 patients, haemoglobin levels decreased in 6/7 patients, LDH levels increased in 4/7 patients, FERR ferritin (FERR) levels increased in 3/7 patients, and alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) levels increased in 5/7patients. Antibody testing for anti-EBV in the serum and detection of EBV DNA in the blood were available for six (patients 1, 2, 3, 4, 6, and 7). EBV-CA-IgM positivity was observed in three patients (patients 1, 2, and 7), and EBV-VCA-IgG positivity was found in six patients. These patients exhibited increased EBV DNA levels. The karyotype was detected in three patients, and no abnormalities were found (patients 1, 5, and 7). None of the patients had a history of hepatitis B or C infection, and no other immunodeficiency diseases were found. Four patients presented with hemophagocytic lymphohistiocytosis HLH (patients 2, 4, 6, and 7).
Morphological features
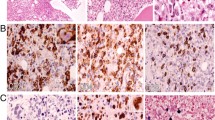
The histopathological changes in the seven patients are summarised in Table 2. The lymph nodes were removed from all samples for biopsy. The architecture was mostly destroyed, and a portion of the lesions involved the peripheral tissue of the node (Fig. 2A). The cortex and medulla of the node were indistinct, and a few residual follicles and subcapsular sinuses that existed were challenging to discern. The cell compositions were diverse. Furthermore, neutrophilic granulocytes, eosinophils, and plasma cells were identified at high magnification. The lesions exhibited numerous infiltrating lymphocytes with medium to large sizes, round to mildly irregular nuclei, inconspicuous or small nucleoli, and dispersed chromatin. Mitotic figures were readily detected, and most cells exhibited significant atypia (Fig. 2B). The lesions were diffuse, with focal-to-extensive coagulative necrosis (Fig. 2C). Additionally, lymphocyte infiltration into the vascular walls was observed.
Immunohistochemical and in situ hybridisation findings
The immune phenotype and EBV infection characteristics of the seven patients are summarised in Table 2; the majority of lymphocytes in the lesion expressed CD3 and TIA-1 or Granzyme B (Fig. 3A, B) but not CD5 (Fig. 3C) in all cases. CD56 expression in the same area was seen in five cases (Fig. 3D). Only a few scattered lymphocytes express CD20. The number of CD8-positive cells (Fig. 3E) was higher than that of CD4-positive cells in six cases. One patient (case 2) had a reduced number of CD4- and CD8-positive cells, and the degree of reduction was similar for both cell types. Ki-67 staining was positive in more than 50% of the cells, except in case 2, where only 40% positivity was observed. The larger scattered cells were CD30-positive in four cases (cases 1, 3, 6, and 7) and LMP1-positive cells in two cases (cases 3 and 5). EBNA2-positive cells were observed in four cases (cases 1, 2, 4, and 7; Fig. 3F). EBER in situ hybridisation revealed a positive signal localisation in the nucleus. EBER-positive cells were observed in the CD3 + /CD56 + areas in five cases, while the other two cells were observed in the CD3 + /CD56 − areas. No EBER-positive cells were observed in the PAX5 + areas in all cases. More than 50 EBER-positive cells per high-power field (> 50/HPF) were observed, with varying cell sizes (Fig. 4A). Double staining for EBER/CD3 and EBER/CD20 showed that most EBER-positive cells were located in the CD3 + areas in all cases (Fig. 4B, C), with only scattered EBER-positive cells found in CD20 + cells.
Immunohistochemical features of EBV + T/NK cells from case 4. Atypical cells exhibiting cytoplasmic CD3 (IHC, × 400) (A). Pleomorphic cells were positive for Granzyme B (IHC, × 400) (B). CD5-positive cells less than CD3 (IHC, × 400) (C). Atypical cells showing strong CD56 staining (IHC, × 400) (D). Almost all cells showed strong granular staining for CD8 (IHC, × 400) (E). EBNA2 showed scattered positive in EBER + cells (IHC, × 800) (F)
EBER and double staining of EBV + cells from case 2. In situ hybridisation of EBV-encoded RNA (EBER). In these lesions, almost all irregular cells showed nuclear labelling (IHC, × 400) (A). EBER-positive cells (brown) with CD3 expression (red) (× 800) (B). EBER-positive cells (brown) were scattered among CD20 + cells (red) (× 800) (C, green arrow)
PCR for TCR gene rearrangements
T-cell clonality analysis by BIOMED-2 PCR revealed that four cases (cases 1, 2, 5, and 6) presented a monoclonal rearrangement, but the other two cases were not tested (Table 2).
Follow-up
Follow-up data were available for all seven patients. Four patients (cases 2, 4, 6, and 7) underwent anti-hemophagocytic lymphohistiocytosis (HLH) therapy, whereas the others received anti-inflammatory treatment. All patients survived. The follow-up duration since diagnosis was 36–84 months (mean duration of 48.7 months). Follow-up CT showed no clear mass or enlarged lymph nodes (Fig. 1B, case 5). The symptoms of all patients were relieved, with no fever, and the lymph node, liver, spleen, and blood test results were within the normal range.
Discussion
EBV-positive lymphoproliferative diseases encompass a range of conditions, including both reactive and malignant disorders. In this study, we examined seven patients diagnosed with EBV + T/NK cells in the IM. All patients survived and are currently under long-term clinical observation and follow-up. It is important to recognise this disease and differentiate it from NK/T-cell lymphoma, systemic EBV + T-cell lymphoma in children, and HLH presenting with extensive infection of T and NK cells. It is important to recognise this complication, and the differential diagnoses include NK/T-cell lymphoma, systemic EBV + T-cell lymphoma in childhood, and HLH.
IM is an acute disease, primarily caused by EBV infection, predominantly affecting B cells, although rare cases involve T cells [11] and NK cells. It typically manifests with high fever, pharyngitis, and cervical lymphadenopathy and often resolves spontaneously. Microscopically, lymph nodes show obvious paracortical expansion and focal destruction of the lymph node architecture. The infiltrating cells are heterogeneous and include small and large lymphocytes, immunoblasts, histiocytes, and variable numbers of plasma cells and eosinophils [12]. The histological features of IM are so varied that they are sometimes misdiagnosed as malignant lymphoma, particularly in cases with numerous large immunoblasts. The clinical symptoms and prognosis described in our present cases were similar to those of IM, except for differences in the types of EBV-infected cells and certain morphological characteristics. While there were two reports describing an IM patient with atypical T-cell proliferation in the nasopharynx, most lymphoid cells were also positive for CD2, CD3, CD5, CD7, and without loss of T-cell antigen [8, 9]. In the present case, the clinical manifestations and blood EBV antibody test results of some patients were consistent with IM; however, excessive EBV infection of T/NK cells has raised concerns about lymphoma. The staining of EBNA2 helps determine the type of EBV infection in pathology, thereby avoiding overdiagnosis of lymphoma. In the IM, T and NK cells may occur, and these might even be monoclonal [13].
NK/T-cell lymphoma occurs mostly in adults, is invasive, progresses rapidly, and almost always exhibits an extranodal presentation. The nasal cavity, nasopharynx, paranasal sinuses, and palate are most commonly involved, with the nasal cavity being the prototypic site of involvement [5]. Some cases may also be accompanied by secondary lymph node involvement [14,15,16]. Isolated nodal involvement in NK/T-cell lymphoma is extremely rare, and only a few cases have been described in published work [17,18,19,20,21]. NK/T-cell lymphoma is highly aggressive, with short survival times and poor response to therapy; therefore, the clinical outcomes are dismal. Differentiating between EBV + T/NK cells in IM and NKTL based on morphology and immunophenotype is difficult. However, NK/T-cell lymphoma often lacks systematic clinical manifestations at onset and progresses rapidly, causing significant damage to adjacent tissue structures. At the same time, we observed positive EBER staining in cells of varying sizes in our cases, suggesting that EBV infection affected cells at different stages of differentiation. The self-limiting characteristic of EBV + T/NK cells in IM underscores its clinical significance. When managing such conditions, careful considerations and selection of treatment strategies are paramount.
Systemic EBV + T-lymphoma in children, according to the WHO classification [5] and Category B classification by Ohshima et al. [7], develops shortly after primary or acute EBV infection and is accompanied by an aggressive clinical course and atypical lymphoid cell infiltration, with most reported cases exhibiting a monoclonal pattern of T-cell proliferation, progressing toward multiple organ failure, sepsis, and sudden death [22, 23]. These malignant tumour features overlapped with those of our present cases in terms of clinical manifestations, pathological morphology, immune phenotype, and clone detection; however, the present patients achieved remission or recovered without relapse during long-term clinical observation. A similar group of cases has been reported [24]; however, the patients died quickly, which differs from our study results. The presence of an abnormal karyotype favours neoplastic conditions and is helpful in identifying systemic EBV + T-lymphoma in children [25]. In the present case, karyotype testing of the three patients showed no abnormalities. The response to HLH treatment is one of the most important parameters for distinguishing IM to Systemic EBV + T-cell lymphoma in childhood.
In the present cases, four patients mimicked EBV-associated HLH, which was described as young age and EBV + T/NK cell proliferation with variable clinical findings, including high fever and splenomegaly, cytopenia, and liver dysfunction, and was accompanied by histological evidence of hemophagocytosis, which causes extremely high serum levels of ferritin, lactate dehydrogenase, and soluble CD25, serological tests, or the detection of EBV DNA or RNA from tissues [26]. After anti-HLH treatment, 4 patients achieved complete remission. The other three patients did not exhibit HLH. In addition to observation and follow-up, symptom relief was achieved without any special treatment. EBV-associated hemophagocytic lymphohistiocytosis (HLH), a hyperinflammatory syndrome induced by a dysregulated immune reaction secondary to EBV infection, is difficult to differentiate in childhood. Clonal EBV-infected T cells have been demonstrated in some cases [13], and patients typically respond to treatment, which can be limited in some cases, with complete recovery following acute disease. Therefore, EBV + T/NK cells in IM can be considered a clinical manifestation of HLH, and the prognosis is generally good after treatment.
Primary EBV-positive nodal T-cell or NK-cell lymphomas have been reported [5, 20, 27]. These lymphomas typically present with lymphadenopathy with or without extranodal involvement, advanced-stage disease, and B symptoms. They exhibit a monomorphic pattern of infiltration and lack of angiodestruction and necrosis seen in extranodal NK/T-cell lymphomas. They are more common in elderly patients or patients with immune deficiency and have a dismal prognosis. Although its immunophenotype is similar to that of EBV + T/NK cells in the IM, it often occurs in the lymph nodes. However, it is more common in elderly patients and has a poor prognosis, which differs from the present case.
It should be noted that a positive monoclonal population does not necessarily predict malignant behaviour because similar populations can be observed under reactive conditions [28,29,30,31]. We identified a clonal T-cell population in four cases (cases 1, 2, 5, and 6). Clonality does not necessarily indicate malignancy, and our results showed that monoclonal T-cell populations are visible in patients with primary EBV infection, consistent with previous reports [13]. Furthermore, the prevention of serious complications such as multiple organ failure, hemophagocytic syndrome, disseminated intravascular coagulation, and sepsis is very important.
It is pertinent to perform a detailed laboratory examination of either EBV load in the serum or expression of EBV in situ to evaluate cases with a sudden onset course. In the present case, serological studies were incomplete or had not been performed. EBV-CA-IgM positivity was observed in three patients (patients 1, 2, and 7), EBV-CA-IgG positivity was observed in six patients, and six patients showed increased EBV DNA. EBV serology in such cases may be clinically misleading, as it may not conclusively indicate acute primary or active infection. However, the precipitous onset of symptoms in previously healthy young individuals is along with biopsy samples showing infiltration of numerous EBER-positive and scattered EBNA2-positive cells suggesting an acute process. In particular, CD8-positive cells increased, suggesting primary EBV infection. EBNA2 (Epstein-Barr virus nuclear antigens 2) is a gene product expressed during latent EBV infection. As a key transcription factor, it regulates the expression of viruses and many genes in cells during viral infection of lymphocytes [11]. Furthermore, EBNA-2 was expressed in four cases with scattered positive nuclei, three of which had increased anti-EBV VCA IgM antibodies. EBNA2 is a useful marker expressed in patients who have a primary immune response or are immunocompromised; IM patients usually have EBNA2-positive cells [9]. Additionally, in the present cases, the indices of liver function and other markers of illness severity in EBV infection were either incomplete or had not been evaluated previously. Therefore, patients with a high viral load in their blood and particularly intense expression of EBV in their vital organs require more intense clinical supervision.
EBV + T/NK cells in the IM can elicit both pathologically malignant and clinically benign features. Thus, detailed clinical information including age at onset (children and young people), nature of onset (sudden), disease course (short), symptoms (systemic), EBV infection status (acute), and lymph node involvement is crucial for the diagnosis and prognostic evaluation of this disease.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or its supplementary materials].
References
Epstein MA, Achong BG, Barr YM (1964) Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet 1:702–703
Auwaerter PG (2006) Recent advances in the understanding of infectious mononucleosis: are prospects improved for treatment or control? Expert Rev Anti Infect Ther 4:1039–1049
Anagnostopoulos I, Hummel M, Kreschel C et al (1995) Morphology, immunophenotype, and distribution of latently and/or productively Epstein-Barr virus-infected cells in acute infectious mononucleosis: implications for the interindividual infection route of Epstein-Barr virus. Blood 85:744–750
Park S, Ko YH (2014) Epstein-Barr virus-associated T/natural killer-cell lymphoproliferative disorders. J Dermatol 41:29–39
Swerdlow SH, Campo E, Harris NL et al (2017) WHO classification of tumours of haematopoietic and lymphoid tissues. IARC, Lyon, pp 360–362
Quintanilla-Martinez L, Kumar S, Fend F et al (2000) Fulminant EBV(+) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood 96:443–451
Ohshima K, Kimura H, Yoshino T et al (2008) Proposed categorization of pathological states of EBV-associated T/natural killer-cell lymphoproliferative disorder (LPD) in children and young adults: overlap with chronic active EBV infection and infantile fulminant EBV T-LPD. Pathol Int 58:209–217
He HL, Wang MC, Huang WT (2013) Infectious mononucleosis mimicking malignant T-cell lymphoma in the nasopharynx: a case report and review of the literature. Int J Clin Exp Pathol 6:105–109
Xie JL, Huang Y, Zheng YY et al (2019) Acute Epstein-Barr virus-positive cytotoxic T cell lymphoid hyperplasia in the upper aerodigestive tract, mimicking extranodal natural killer/T cell lymphoma, nasal type. Virchows Arch 474:219–226
van Dongen JJ, Langerak AW, Bruggemann M et al (2003) Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 17:2257–2317
Klein G, Klein E, Kashuba E (2010) Interaction of Epstein-Barr virus (EBV) with human B-lymphocytes. Biochem Biophys Res Commun 396:67–73
Strickler JG, Fedeli F, Horwitz CA et al (1993) Infectious mononucleosis in lymphoid tissue. Histopathology, in situ hybridization, and differential diagnosis. Arch Pathol Lab Med 117:269–278
Barros MHM, Vera-Lozada G, Segges P et al (2019) Revisiting the tissue microenvironment of infectious mononucleosis: identification of EBV infection in T cells and deep characterization of immune profiles. Front Immunol 10:146
Huang YH, Wu QL, Zong YS et al (2011) Nasopharyngeal extranodal NK/T-cell lymphoma, nasal type: retrospective study of 18 consecutive cases in Guangzhou, China. Int J Surg Pathol 19:51–61
Jo JC, Yoon DH, Kim S et al (2012) Clinical features and prognostic model for extranasal NK/T-cell lymphoma. Eur J Haematol 89:103–110
Kim SJ, Oh SY, Hong JY et al (2010) When do we need central nervous system prophylaxis in patients with extranodal NK/T-cell lymphoma, nasal type? Ann Oncol 21:1058–1063
Gualco G, Domeny-Duarte P, Chioato L et al (2011) Clinicopathologic and molecular features of 122 Brazilian cases of nodal and extranodal NK/T-cell lymphoma, nasal type, with EBV subtyping analysis. Am J Surg Pathol 35:1195–1203
Jeon YK, Kim JH, Sung JY et al (2015) Epstein-Barr virus-positive nodal T/NK-cell lymphoma: an analysis of 15 cases with distinct clinicopathological features. Hum Pathol 46:981–990
Chang ST, Liao YL, Lin SH et al (2010) NK-cell lymphoma with nodal presentation and expression of cutaneous lymphocyte-associated antigen. Pathol Res Pract 206:463–466
Chim CS, Ma ES, Loong F et al (2005) Diagnostic cues for natural killer cell lymphoma: primary nodal presentation and the role of in situ hybridisation for Epstein-Barr virus encoded early small RNA in detecting occult bone marrow involvement. J Clin Pathol 58:443–445
Takahashi E, Asano N, Li C et al (2008) Nodal T/NK-cell lymphoma of nasal type: a clinicopathological study of six cases. Histopathology 52:585–596
Abdul-Ghafar J, Kim JW, Park KH et al (2011) Fulminant Epstein-Barr virus-associated T-cell lymphoproliferative disorder in an immunocompetent middle-aged man presenting with chronic diarrhea and gastrointestinal bleeding. J Korean Med Sci 26:1103–1107
Young KH, Zhang D, Malik JT et al (2008) Fulminant EBV-driven CD8 T-cell lymphoproliferative disorder following primary acute EBV infection: a unique spectrum of T-cell malignancy. Int J Clin Exp Pathol 1:185–197
Rodríguez-Pinilla SM, Barrionuevo C, García J et al (2011) Epstein-Barr virus-positive systemic NK/T-cell lymphomas in children: report of six cases. Histopathology 59:1183–1193
Hue SSS, Oon ML, Wang S et al (2020) Epstein-Barr virus-associated T- and NK-cell lymphoproliferative diseases: an update and diagnostic approach. Pathology 52:111–127
Kim WY, Montes-Mojarro IA, Fend F et al (2019) Epstein-Barr virus-associated T and NK-cell lymphoproliferative diseases. Front Pediatr 15:71
Wang GN, Zhao WG, Zhang DD et al (2020) Clinicopathological features of primary EB virus positive nodal T/NK cell lymphoma. Zhonghua Bing Li Xue Za Zhi 49(10):1009–1014. https://doi.org/10.3760/cma.j.cn112151-20200213-00087
Kim Y, Choi YD, Choi C et al (2013) Diagnostic utility of a clonality test for lymphoproliferative diseases in Koreans using the BIOMED-2 PCR assay. Korean J Pathol 47:458–465
Lee HY, Baek JO, Lee JR et al (2012) Atypical hydroa vacciniforme-like Epstein-Barr virus associated T/NK-cell lymphoproliferative disorder. Am J Dermatopathol 34:e119–e124
Callan MF, Steven N, Krausa P et al (1996) Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat Med 2:906–911
Bahler DW, Swerdlow SH (1998) Clonal salivary gland infiltrates associated with myoepithelial sialadenitis (Sjögren’s syndrome) begin as nonmalignant antigen-selected expansions. Blood 91:1864–1872
Acknowledgements
The authors thank Dr. Sun Jirui for providing clinical follow-up details and Baoding First Central Hospital, Hebei Province, and Dr. Teng Xiaojing’s guidance for the technical staff of the double staining at the Capital Medical University, Beijing Friendship Hospital.
Funding
This study received funding from the National Key R&D Program of China (2020YFC2004800).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All procedures were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and the Helsinki Declaration of 1964 and its later versions.
Informed consent
Informed consent was obtained from all the participants. Ethical approval was obtained from the Ethical Review Board of the Beijing Friendship Hospital, Capital Medical University (No. 2019-P2-006–001).
Consent for publication
Consent for publication was obtained from all the individuals included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Xie, J., Zheng, Y. et al. Infectious mononucleosis complicated by transitory Epstein-Barr virus infection of T and natural killer cells. J Hematopathol (2024). https://doi.org/10.1007/s12308-024-00595-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12308-024-00595-6