Abstract
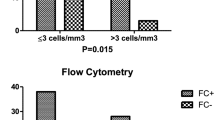
Central nervous system (CNS) involvement is a serious complication in hematologic malignancy, and early detection and management of CNS involvement in these cases significantly impact the prognosis. Currently, there is no consensus on the use of multiparametric flow cytometry (MFC) and conventional cytology (CC) testing for initial and follow-up cerebrospinal fluid (CSF) specimens to diagnose CNS involvement by hematologic malignancy. In our institution, after initial MFC and CC, two subsequent negative MFCs are required before discontinuing MFC. The aim of this study is to evaluate the outcome of this approach. CSF cytology and MFC reports were retrieved from Laboratory Information System, and data was reviewed. Between January 2020 and December 2021, 1789 CSF samples from 280 patients were submitted for CSF analysis. For those 517 CSF samples tested by both MFC and CC, 97 cases tested positive by both MFC and CC with 95% concordance. Eighteen cases were MFC + /CC − and 7 were MFC − /CC + . Thirty-six cases had initially positive MFCs followed by more than one MFC evaluation. Among those 36 cases, 22 cases (61.1%) converted to negative after the second follow-up sample, 9 cases (25%) were continuously positive for at least three samples, and 5 cases (13.9%) exhibited negative to positive conversion. Compared to negative CSF cases, positive CSFs had higher total nucleated cell count and higher total protein levels while red blood cells, glucose, and lactate dehydrogenase levels remained at comparable levels. The concordance between MFC and CC was excellent. The high incidence of positive MFCs on two or more follow-up samples and the high frequency of negative MFC to positive conversion indicate the necessity of repeated negative MFCs before discontinuing MFC. The fact that more than half of the positive cases converted to negative after the second CSF specimen and most follow-up positive cases can be detected by CC alone suggests it is adequate to use CC alone for follow-up CSF study after two consecutive negative MFCs.



Similar content being viewed by others
Change history
05 July 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12308-023-00553-8
References
Del Principe MI, Gatti A, Johansson U et al (2021) ESCCA/ISCCA protocol for the analysis of cerebrospinal fluid by multiparametric flow-cytometry in hematological malignancies. Cytometry B Clin Cytom 100(3):269–281
Haioun C, Besson C, Lepage E et al (2000) Incidence and risk factors of central nervous system relapse in histologically aggressive non-Hodgkin’s lymphoma uniformly treated and receiving intrathecal central nervous system prophylaxis: a GELA study on 974 patients. Groupe d’Etudes des Lymphomes de l’Adulte. Ann Oncol. 11(6):685–90
de Graaf MT, de Jongste AH, Kraan J et al (2011) Flow cytometric characterization of cerebrospinal fluid cells. Cytometry B Clin Cytom 80(5):271–281
Chamberlain MC, Johnston SK, Van Horn A et al (2009) Recurrent lymphomatous meningitis treated with intra-CSF rituximab and liposomal ara-C. J Neurooncol 91(3):271–277
Bromberg JE, Breems DA, Kraan J et al (2007) CSF flow cytometry greatly improves diagnostic accuracy in CNS hematologic malignancies. Neurology 68(20):1674–1679
Mitri Z, Siddiqui MT, El Rassi F et al (2014) Sensitivity and specificity of cerebrospinal fluid flow cytometry for the diagnosis of leukemic meningitis in acute lymphoblastic leukemia/lymphoma. Leuk Lymphoma 55(7):1498–1500
Cesana C, Klersy C, Scarpati B et al (2011) Flow cytometry and cytomorphology evaluation of hematologic malignancy in cerebrospinal fluids: comparison with retrospective clinical outcome. Ann Hematol 90(7):827–835
Alvarez R, Dupuis J, Plonquet A et al (2012) Clinical relevance of flow cytometric immunophenotyping of the cerebrospinal fluid in patients with diffuse large B-cell lymphoma. Ann Oncol 23(5):1274–1279
Au KLK, Latonas S, Shameli A et al (2020) Cerebrospinal fluid flow cytometry: utility in central nervous system lymphoma diagnosis. Can J Neurol Sci 47(3):382–388
Fox CP, Phillips EH, Smith J et al (2019) Guidelines for the diagnosis and management of primary central nervous system diffuse large B-cell lymphoma. Br J Haematol 184(3):348–363
Tawfik B, Brown L, Fuda F et al (2018) Utility and proposed algorithm of CSF flow cytometry in hematologic malignancies. Ann Hematol 97(9):1707–1716
Jaime-Perez JC, Borrego-Lopez MF, Jimenez-Castillo RA et al (2018) Comparison of conventional cytomorphology, flow cytometry immunophenotyping, and automated cell counting of CSF for detection of CNS involvement in acute lymphoblastic leukemia. Int J Lab Hematol 40(2):169–174
Chow E, Troy SB (2014) The differential diagnosis of hypoglycorrhachia in adult patients. Am J Med Sci 348(3):186–190
Schwenkenbecher P, Janssen T, Wurster U et al (2019) The influence of blood contamination on cerebrospinal fluid diagnostics. Front Neurol 10:584
Szantho E, Karai B, Ivady G et al (2019) Evaluation of sample quality as preanalytical error in flow cytometry analysis in childhood acute lymphoblastic leukemia. EJIFCC 30(4):385–395
Bento LC, Correia RP, Alexandre AM et al (2018) Detection of central nervous system infiltration by myeloid and lymphoid hematologic neoplasms using flow cytometry analysis: diagnostic accuracy study. Front Med (Lausanne) 5:70
Nam AS, Giorgadze T, Tam W et al (2018) Assessment of the utility of cytology and flow cytometry of cerebrospinal fluid samples in clinical practice. Acta Cytol 62(2):130–136
Rocha JMC, Murao M, Cancela CSP et al (2021) Comparative analysis between cytomorphology and flow cytometry methods in central nervous system infiltration assessment in oncohematological patients. Hematol Transfus Cell Ther p 1–8. https://doi.org/10.1016/j.htct.2021.09.016
Scharf EL, Hanson CA, Howard MT et al (2016) Serial cerebrospinal fluid examinations to diagnose hematological malignancy causing neurological disease. J Neurooncol 129(1):77–83
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards, with the Human Investigation Committee (IRB) approval (I 3324822) at our institution.
Informed consent
This chart review study does not reveal Protected Health Information (PHI) or identifying details such as names, dates of birth, identity numbers, biometrical characteristics (such as facial features, fingerprint, writing style, voice pattern, DNA, or other distinguishing characteristic). No informed consent was obtained from individuals included in the study.
Consent for publication
This chart review study does not reveal Protected Health Information (PHI) or identifying details such as names, dates of birth, identity numbers, biometrical characteristics (such as facial features, fingerprint, writing style, voice pattern, DNA or other distinguishing characteristic). No informed consent was obtained from individuals included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In this article the graphics relating to Figs. 2 and 3 captions had been interchanged; the figure(s) should have appeared as shown below.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Momen, N., Tario, J., Fu, K. et al. Initial and follow-up evaluations on cerebrospinal fluid involvement by hematologic malignancy. J Hematopathol 16, 131–140 (2023). https://doi.org/10.1007/s12308-023-00550-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-023-00550-x