Abstract
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL) and comprises a heterogeneous group of disease. While classification of B-cell lymphomas has been evolving to include clonality in a specific manner, morphology, and immunohistochemistry remain the backbone. We aimed to evaluate the value of CD5 expression on disease characteristics as well as prognosis in patients with DLBCL. Data of 131 patients with DLBCL with CD5 positivity and as a comparison arm, data of 129 patients with DLBCL without CD5 positivity were evaluated. Mean age was 59 and 55.7% of the patients were male. Overall survival was 29.8 months. Poor prognostic factors including (high-LDH levels, B symptoms, low ECOG score, high R-IPI and NCCN-IPI score) were observed to be significantly related with CD5 positivity. Mean survival in CD5 positive patients were 29.8 months, which is significantly shorter than the general DLBCL survival worldwide. CD5 expression shall be evaluated in all samples of DLBCL patients due to its possible effects on outcomes.
Similar content being viewed by others
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma (NHL), accounting for almost 40% of patients. With the introduction of molecular assessment, DLBCL has been recognized as a conceptual diagnosis with various subtypes. While the first-line treatment has not been changed, complete remission with first-line treatment being 60% [1–3] remains as the main concern, and identification of high-risk patients who may poorly response to first-line treatment is now the main goal of care [4, 5].
While the classification of DLBCL is a still evolving concept, CD5 expression in DLBCL has been recognized as a distinct entity with conflicting importance. CD5 positivity has been observed with a range of 5% and 22% in newly diagnosed DLBCL [6, 7]. In DLBCL patients who had CD5 expression, this population, unlike the general population of DLBCL patients, LDH enzyme increase, extra nodal involvement, bone marrow involvement, poor ECOG (Eastern Cooperative Oncology Group) performance score, poor international prognostic score, and female tendency were observed to be more frequent. As expected, worse prognosis was observed in patients with CD5 positivity ( +) DLBCL, as well as a higher relapse rate were observed compared to CD5 negative ( −) DLBCL patients [7, 8].
Since CD5 is a T lymphocyte marker and not essential in the diagnosis of DLBCL, it is often neglected in the diagnostic work up leading to conflicting outcomes in terms of its importance. We aimed to determine the frequency of CD5 expression and its association with disease outcomes in DLBCL.
Methods
The study was conducted by the Lymphoma Scientific Subcommittee of Turkish Society of Hematology, including 16 centers among 9 cities that represent multiple geographic areas of Turkey. Diagnostic pathology reports of DLBCL patients between 2015 and 2022 were evaluated in a retrospective manner.
Primary central nervous system (CNS) lymphoma patients were excluded due to poor prognosis and their divergent first-line treatment. Within 2469 patients, CD5 positivity was observed in 169 patients. Complete data of 131 CD5-positive patients and 129 CD5-negative patients were available. Patients’ age, gender, date of diagnosis, stage at diagnosis (Ann-Arbor), ECOG performance evaluation, LDH levels, R-IPI and NCCN-IPI scores, extra nodal and bone marrow involvement, treatments modalities, response to treatment, and follow-up were recorded from the patient files. Pre-treatment samples of all patients were evaluated.
This study was approved by Trakya University Faculty of Medicine Ethical Committee (TUTF-BAEK2020/439).
Pathological assessment and definitions
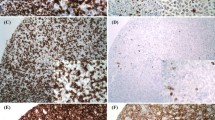
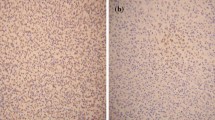
As a routine practice, pathological assessment of lymph nodes was performed on formalin-fixed, paraffin-embedded tissue sections. A panel of selected antibodies was used by the pathologists: CD20, CD3, CD5, CD10, BCL-2, BCL-6, MUM1, FOXP-1, Ki-67, and MYC proto-oncogene protein (MYC). A cutoff level for dim to strong expression of CD5 was ≥ 50% of tumor cells for considering as immunopositivity. Patients were grouped as germinal center and non-germinal center DLBC based on the Hans algorithm using CD10, BCL-6, and MUM-1 [9]. The cutoffs for positive MYC or BCL-2 results were ≥ 40% and ≥ 50% of cells (Fig. 1) [10, 11].
Fluorescence in situ hybridization (FISH) was performed on cases using LSI dual-color break-apart probes for MYC and BCL6, and dual-color, dual-fusion probe for BCL2/IGH. Positive cases for rearrangements had positive signal in ≥ 15% of nuclei examined.
Statistics
Normality assumption was assessed using Shapiro–Wilk test. Student’s t test and one-way analysis of variance were used to compare independent groups. Tukey post hoc test was used to perform multiple comparisons. The log-rank test using the Kaplan–Meier method was performed for univariate survival analysis. The Cox proportional hazards model with a forward selection method was used to identify the prognostic significance of risk factors on survival. Descriptive statistics were expressed as mean and standard deviation for numerical variables and frequency and percentage for categorical variables. All statistical analyses were performed using SPSS version 20 (SPSS software, IBM Corporation, Armonk, NY).
Results
Records of 2469 DLBCL patients were evaluated for the study. CD5 staining was done for all samples and CD5 positivity was observed in 169 patients (6.84%). Complete data of 131 CD5-positive patients and 129 CD5-negative patients were available and considered for further comparisons. In CD5 positivity group, the mean age was 59 years (18–88). Fifty-five percent of the patients were 60 years and older. A 55.7% of the patients were male. In CD5-negative group, the mean age was 64.4 years (25–93). Forty-five percent of the patients were 60 years and older. A 54.2% of the patients were male. In CD5 positivity group, overall survival was 29.8 months (0.1–72.3 months), and it was found to be statistically shorter than CD5-negative patients (OS was 25 months (0.1–70 months)) (p = 0.01) (Fig. 2). Progression-free survival (PFS) was 26.08 months. 41 of 131 (31.3%) patients died during follow-up. General characteristics of patients are summarized in Table 1.
In CD5 positivity group 76 patients were non-GCB (61.7%) while 43 patients were GCB (34.9%) and 4 patients were unclassified. In the CD5-negative group, only 36.4% of patients were non-GCB (p = 0.00). When the CD5 -positive group is examined, LDH was observed to be elevated in 65.4% of the patients. Eighty-four patients (66.7%) had Ann-Arbor stage 3–4 disease. Seventy percent of the patients had 1 or more extra nodal involvement. R-IPI score was high in 48% of patients, and NCCN-IPI was intermediate-high or high in 44.1% of the patients. However, in CD5-negative group, only 34.8% of the patients had high LDH values (p = 0.412) and 56.5% of the patients had Ann-Arbor stage 3–4 disease. In addition, only 30% of the patients had extranodal involvement, which was found to be much less than the patients in the CD5 -positive group (p = 0.00). Similarly, the rate of patients with high R-IPI (20.5%) was found to be much lower than those of CD5-positive patients (p = 0.00). In CD5 positivity group, the mean Ki67 was 78.15%. ECOG performance score was observed to be ≥ 2 in 31.25% of the patients. 57% patients had B symptoms (Table 1).
Evaluation of prognostic factors in CD5-positive patients
The mean survival was significantly longer in patients under 60 years (63.41 vs. 42.81 months, p < 0.01). There was no difference in survival between genders (p = 0.891). Patients with B symptom at the time of diagnosis (57%) had a shorter survival (p = 0.002) (Fig. 2). The mean survival of patients with bone marrow involvement was statistically significantly lower (32.03–58.27 months, p < 0.01), likewise in patients with extra nodal involvement (46.90 vs. 66.23 months, p = 0.02) (Figs. 3–4).
Increased LDH was observed to be related with survival (p < 0.01). A 27.5% of the patients had a worse performance status than ECOG 1 and performance was significantly related with survival (p < 0.01). Survival was poorer in patients with both high-intermediate and high risk NCCN-IPI and high R-IPI (> 3) scores (p < 0.01) (Figs. 4–5).
Regarding first-line treatment, 11 patients have received more intense treatment including dose-adjusted EPOCH-R, R-CHOEP, or hyper-CVAD and survival of these patients were similar with patients who received R-CHOP (p = 0.997) (Table 2).
Multivariate analysis was performed by excluding R-IPI and NCCN-IPI, which are among the other data, being ≥ 60 years of age, ECOG ≥ 2 and ≥ 1 extra nodal involvement were related with overall survival as well progressive free survival (Figs. 6, 7, and 8).
Discussion
As a pan T-cell marker, CD5 is a monomeric type 1 transmembrane glycoprotein. It has both an intracellular and extracellular domain. Although it is frequently expressed from T cells, it is also expressed at a low level by a small subset of naive B lymphocytes [12]. CD5 inhibits signaling downstream of the B-cell receptor and modifies intracellular calcium mobilization. It also suppresses the release of IL-2, resulting in increased production of IL-10, an anti-inflammatory marker and a survival factor for B cells. Additionally, activation of the ERK1/2, PI3K, STAT3, and NFAT2 signal pathways were observed in CD5 positive B cells [7, 12]. CD5-positive B cells may also produce autoantibodies, which may be related with the development of autoimmune events in chronic lymphocytic leukemia [13]. Factors with prognostic relevance including high-LDH levels, bone marrow involvement, presence of extra nodal involvement, high-IPI scores, poor ECOG performance, and CNS recurrence have been more frequently observed in patients with CD5 + DLBCL [8].
The frequency of CD5 positivity is variable in de novo DLBCL. In a retrospective study about the characteristics of CD5 + DLBCL patients in Korea, CD5 positivity was found to be 7.4% [14]. In a multicenter study by Yamaguchi et al. in Japan, this frequency was reported as 10% [15]. However, in large-scale studies in Japan, prevalence rates of CD5 positivity may vary widely (5–22%) [7, 15]. Besides these, in a multicenter large-scale study with data from Western countries, CD5 positivity ratio in DLBCL is (5.5%) [16]. We observed the ratio as 6.84% which is similar in Western countries but dissimilar to East Asian countries. Immunohistochemical CD5 staining not being a routinely performed in all samples may be the reason for the conflicting frequencies across countries and centers. Although, there is a predominance of female patient distribution in CD5 + DLBCL patients in studies from East Asian countries, we have observed a male gender tendency (55.7%) similar with Western countries. Based on the latest classification proposed by World Health Organization and International Consensus Classification Advisory Committee, CD5 positivity was not regarded as a prognostic marker in patients with DLBCL. This may be due to the small percentage of CD5 positivity in this group of patients and may be altered in future classifications [17, 18].
In DLBCL patients, remission rate with current first-line treatment is 50–60%. Based on prognostic scores, the 5-year survival in patients with low-IPI score is 90%, while survival is observed to decrease to 60% in patients with high-IPI scores. Based on the cell of origin, 5-year overall survival (OS) was 48% to 56% in non-GCB subtype, while 73% to 78% in GCB subtype. The 5-year OS in the DHT/THT subtype appears to be very low and nearly 18% [1, 19]. The 5-year OS is 35.5% in CD5 + DLBCL patients, and it has been found to be shorter than general DLBCL patient population [12]. In our study, we observed that the mean survival was 29.8 months in CD5 + DLBCL patients, similar with the literature.
In addition to the shorter OS of CD5 + DLBCL patients, these patients have higher LDH, IPI elevation, advanced Ann-Arbor frequency, poor performance score, and bone marrow involvement at the time of diagnosis compared to CD5-DLBCL patients. CD5 + DLBCL is more often in the non-GCM/ABC phenotype. Also, the presence of double/triple expressor or double/triple hits are more common in these patients at the time of diagnosis [12, 14–16, 20]. Non-GCM/ABC phenotype as well as other factors related with poor prognosis and worse outcomes being more frequent in CD5 positive groups, we may assume that this very group of disease may be regarded as relatively high risk.
The age and gender distribution at the time of diagnosis is variable. In studies from East Asian countries, the frequency of female patients is significantly higher [12, 14, 15]. However, there is no difference in gender distribution in Western countries [16]. In our data, it was observed that male patients were in the majority (55.7%). In the study conducted on CD5 + DLBCL patient data from western countries, it is observed that 76.7% of CD5 + patients are over 60 years old [16]. But, in a single-center study in China, 53.3% of CD5 + DLBCL patients were below the age of 60 [12]. In our data, the rate of patients, 60 years and older, are higher (55%) (Table 3).and – patients group
Central nervous system relapse is reported to be more frequent in CD5 + DLBCL patients. In the study by Zhang et al., it was observed that CNS involvement in relapsed DLBCL patients was 35% in CD5 + patients, (19% in CD5 −) [20]. Similarly, in the study by Thakral B. et al., CNS relapsed was 33.3% in CD5 + and 15.6% in CD5 − patients [21]. We observed that 19 patients have relapsed and only 4 patients had CNS relapsed (3% of all patients, 21% of all relapses). CNS prophylaxis was not a routine component of the main treatment in all centers and CNS relapse was not related with the use of prophylaxis.
In our data, 64.6% of the patients had high LDH levels (13.4% \(\ge\) threefold). Twenty-three percent of the patients had bone marrow involvement, 70% had extra nodal involvement, and 57% of the patients had B symptoms. Advanced Ann-Arbor stage disease was observed in 66.6% of patients, in addition to the higher frequency of all these factors that have impact on prognosis for CD5 + DLBCL patients. In a study with a small number of patients from Taiwan, the presence of CD5 + was observed as the only adverse prognostic factor determined in multivariate analysis [22].
Increased OS with the introduction of rituximab seems to be valid also for CD 5 + patients. A study by Hyo R et al. showed that addition of rituximab to chemotherapy similarly increased OS and progression-free survival (PFS) [23]. A study by Miyazaki K. et al. revealed that addition of rituximab to chemotherapy in CD 5 + DLBCL patients significantly increases OS [24]. However, even if rituximab is included in the treatment, a prospective study by Tzankov A et al. reported a negative effect of the presence of CD5 in multiparameter analyzes in patients receiving R-CHOP [25]. In a retrospective study, in which DLBCL patients treated with R-EPOCH chemotherapy, with a 28.5-month median follow-up, 37.5% of CD5 + patients had died (9.6% in CD5 −) [21]. In the study conducted by Zhang F. et al. to evaluate the effect of intensive treatment on survival, the effectiveness of da-EPOCH-R and R-CHOP treatments were compared. It was observed that PFS and OS were better in both CD5 + and CD5 − groups in patients who received da-EPOCH-R in short-term follow-up. In the long-term follow-up, this was not observed for OS in CD5 + patients. Also, in another study with a small group of patients with short-term follow-up, we can say that the da-EPOCH-R combined with high-dose methotrexate regimen had a better survival in the CD5 + patient group [26]. We also observed that the first-line treatment of 11 patients was a more intensive treatment (da-EPOCH-R, R-CHOEP, hyper-CVAD). The mean survival of these patients compared to patients who received R-CHOP as first-line therapy was similar. The low number of patients receiving intensive treatment seems to be an important limiting factor in this comparison.
Limitations
The study had two important limiting factors. First, we were able to access to a limited extent to survival statistics of CD5 − control group. The second important limitation was the study being retrospective.
Study highlight
– Due to its prognostic effect, CD5 must be applied immunohistochemically for all DLBCL patients.
– CD5 status and CNS relapse needs to be evaluated in further studies.
– Considering the data in the literature and the data in our study, the different evaluation of CD5 + patients seems to be an indisputable fact.
– With the survival and literature information, we think there is an unmet need for this subgroup of patients with low survival expectancy. Thus, we need new studies with novel treatment modalities to be combined or to replace the standard of care therapies.
References
Liu Y, Barta SK (2019) Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol 94:604–616. https://doi.org/10.1002/ajh.25460
Tilly H, Silva MG, Vitolo U et al (2015) Diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis treatment and follow-up. Ann Oncol 2:116–125. https://doi.org/10.1093/annonc/mdv304
Li S, Young KH, Medeiros J (2018) Diffuse large B-cell lymphoma. Pathology 50(1):74–87. https://doi.org/10.1016/j.pathol.2017.09.006
Jaffe ES, Campo E, Harris NL et al (2017) WHO classification of tumours of haematopoietic and lymphoid tissues. Introduction overwiev of the classification of lymphoid neoplasm, 4th edn. International Agency for Research on Cancer, pp 190–198
Huang S, Nong L, Wang W et al (2019) Prognostic impact of diffuse large B-cell lymphoma with extra copies of MYC, BCL2 and/or BCL6: comparison with double/triple hit lymphoma and double expressor lymphoma. Diagn Pathol 14(1):81. https://doi.org/10.1186/s13000-019-0856-7
Sukswai N, Lyapichev K, Khoury JD, Medeiros LJ (2020) Diffuse large B-cell lymphoma variants: an update. Pathology 52(1):53–67. https://doi.org/10.1016/j.pathol.2019.08.013
Xu Y, Sun W, Li F (2020) De Novo CD5+ Diffuse large B-cell lymphoma: biology, mechanism, and treatment advances. Clin Lymphoma Myeloma Leuk 20(10):e782–e790. https://doi.org/10.1016/j.clml.2020.05.003
Ting CY, Chang KM, Kuan JW et al (2019) Clinical significance of BCL2, C-MYC, and BCL6 genetic abnormalities, Epstein-Barr virus infection, CD5 protein expression, germinal center B cell/non-germinal center B-cell subtypes, co-expression of MYC/BCL2 proteins and co-expression of MYC/BCL2/BCL6 proteins in diffuse large B-cell lymphoma: a clinical and pathological correlation study of 120 patients. Int J Med Sci 16(4):556–566. https://doi.org/10.7150/ijms.27610
Hans CP, Weisenburger DD, Greiner TC et al (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immuno- histochemistry using a tissue microarray. Blood 103:275–282. https://doi.org/10.1182/blood-2003-05-1545
Visco C, Li Y, Xu-Monette ZY et al (2012) Comprehensive gene expression profiling and immunohistochemical studies support application of immunophenotypic algorithm for molecular subtype classification in diffuse large B-cell lymphoma: a report from the International DLBCL Rituximab-CHOP Consortium Program Study. Leukemia 26(9):2103–2113. https://doi.org/10.1038/leu.2012.83
Yoshioka T, Miura I, Kume M et al (2005) Cytogenetic features of de novo CD5- positive diffuse large B-cell lymphoma: chromosome aberrations affecting 8p21 and 11q13 constitute major subgroups with different overall survival. Genes Chromosomes Cancer 42:149–157. https://doi.org/10.1002/gcc.20127
Tang H, Zhou H, Wei J et al (2019) Clinicopathologic significance and therapeutic implication of de novo CD5+ diffuse large B-cell lymphoma. Hematology 24(1):446–454. https://doi.org/10.1080/16078454.2019.1614289
Jain P, Fayad LE, Rosenwald A et al (2013) Recent advances in de novo CD5+ diffuse large B cell lyphoma. Am J Hematol 88(9):798–802. https://doi.org/10.1002/ajh.23467
Na HY, Choe J, Shin SA et al (2019) Characteristics of CD5-positive diffuse large B-cell lymphoma among Koreans: high incidence of BCL2 and MYC double-expressors. PLoS One 14(10):e0224247. https://doi.org/10.1371/journal.pone.0224247
Yamaguchi M, Seto M, Okamoto M et al (2002) De novo CD5+ diffuse large B-cell lymphoma: a clinicopathologic study of 109 patients. Blood 99(3):815–21. https://doi.org/10.1182/blood.v99.3.815
Xu-Monette ZY, Tu M, Jabbar KJ et al (2015) Clinical and biological significance of de novo CD5+ diffuse large B-cell lymphoma in Western countries. Oncotarget 6(8):5615–33. https://doi.org/10.18632/oncotarget.3479
Alaggio R, Amador C, Anagnostopoulos I et al (2022) The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia 36:1720–1748. https://doi.org/10.1038/s41375-022-01620-2
Campo E, Jaffe ES, Cook JR et al (2022) The ınternational consensus classification of mature lymphoid neoplasms: a report from the clinical advisory committee. Blood 140(11):1229–1253. https://doi.org/10.1182/blood.2022015851
Sehn LH, Salles G (2021) Diffuse large B-cell lymphoma. N Engl J Med 384:9. https://doi.org/10.1056/NEJMra2027612
Zhang F, Li L, Zhang L et al (2019) Prognostic analysis of CD5 expression in double-hit diffuse large B-cell lymphoma and effectiveness comparison in patients treated with dose-adjusted EPOCH plus rituximab/R-CHOP regimens. Blood Lymphat Cancer 9:33–43. https://doi.org/10.2147/BLCTT.S216292
Thakral B, Medeiros LJ, Desai P et al (2017) Prognostic impact of CD5 expression in diffuse large B-cell lymphoma in patients treated with rituximab-EPOCH. Eur J Haematol 98(4):415–421. https://doi.org/10.1111/ejh.12847
Chuang WY, Chang H, Shih LY et al (2015) CD5 positivity is an independent adverse prognostic factor in elderly patients with diffuse large B cell lymphoma. Virchows Arch 467(5):571–582. https://doi.org/10.1371/journal.pone.0224247.t003
Hyo R, Tomita N, Takeuchi K et al (2010) The therapeutic effect of rituximab on CD5-positive and CD5-negative diffuse large B-cell lymphoma. Hematol Oncol 28(1):27–32. https://doi.org/10.1002/hon.896
Miyazaki K, Yamaguchi M, Suzuki R et al (2011) CD5-positive diffuse large B-cell lymphoma: a retrospective study in 337 patients treated by chemotherapy with or without rituximab. Ann Oncol 22:1601–1607. https://doi.org/10.1093/annonc/mdq627
Tzankov A, Leu N, Muenst S et al (2015) Multiparameter analysis of homogeneously R-CHOP-treated diffuse large B cell lymphomas identifies CD5 and FOXP1 as relevant prognostic biomarkers: report of the prospective SAKK 38/07 study. J Hematol Oncol 14(8):70
Miyazaki K, Asano N, Yamada T et al (2020) DA-EPOCH-R combined with high-dose methotrexate in patients with newly diagnosed stage II-IV CD5-positive diffuse large B-cell lymphoma: a single-arm, open-label, phase II study. Haematologica 105(9):2308–2315. https://doi.org/10.3324/haematol.2019.231076
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
TUTF-BAEK2020/439
Informed consent
Informed consent was obtained from all subjects as a retrospective study. Also, informed consent was obtained from a parent and/or legal guardian for patients who died.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Demirci, U., Kırkızlar, H.O., Ümit, E.G. et al. CD5 as a prognostic marker in patients with diffuse large B-cell lymphoma: a multicenter study. J Hematopathol 15, 203–213 (2022). https://doi.org/10.1007/s12308-022-00523-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-022-00523-6