Abstract
Measurable residual disease (MRD) testing has become a standard practice for patients with acute myeloid leukemia (AML) following therapy. However, MRD testing in AML is not straightforward, and both molecular methods and multiparameter flow cytometric (MFC) methods demonstrate clinical utility. While MFC methods are potentially applicable to all AML patients, current molecular MRD methods must be individually tailored to the patient’s AML disease genetics, a strategy that is currently applied for patients with acute promyelocytic leukemia, core binding factor AML, and AML with mutated NPM1. However, there is great interest in next-generation sequencing (NGS) methods for MRD assessment, an approach that could potentially be applied to all patients with AML. Current NGS methods have limited analytic sensitivity when compared with other molecular methods and MFC, but advances in NGS methods and informatic algorithms may improve NGS MRD testing in the near future. In this review, we discuss current recommendations for molecular MRD assessment in AML and discuss opportunities and challenges for MRD assessment by NGS methods.
Similar content being viewed by others
Introduction
The evaluation of post-therapy bone marrow specimens from patients with acute myeloid leukemia (AML) has historically relied on morphologic assessment. If fewer than 5% of marrow cells are blasts and if Auer rods (signifying an abnormal blast population) are not identified, then a patient is determined to be in a morphologic leukemia-free state, a prerequisite for complete remission (CR) [1, 2]. However, attainment of morphologic CR does not adequately predict outcomes in patients with AML, and it has become apparent that morphologic absence of leukemia is too crude a metric to guide optimal patient management [3, 4]. Manual differential counting is subjective, and counting relatively few cells (~ 500) leads to substantial measurement error, even under optimal theoretical circumstances, when dealing with low blast percentages [5]. Moreover, given the vast numbers of hematopoietic cells in the body, even 1% malignant blasts in a marrow sample would translate to billions of leukemic cells in the patient [6, 7]. Further, regenerating normal myeloblasts following chemotherapy cannot be reliably distinguished from neoplastic leukemic blasts by morphologic inspection. Therefore, techniques with greater clinical sensitivity than morphologic examination to monitor disease in AML patients are needed. Building upon successes in acute lymphoblastic leukemia (ALL) and chronic myeloid leukemia (CML) [8,9,10], the utilization of non-morphologic modalities to assess for measurable (minimal) residual disease (MRD) in AML has increased over the last several years.
There has been some hesitation to fully embrace MRD testing for patients with AML and to use these results to make treatment-related decisions due to a lack of randomized clinical trials [7]. However, clear associations between AML MRD status and the risk of frank relapse and inferior outcome [11, 12], as well as promising data from prospective non-randomized trials in which MRD positivity was used to guide subsequent therapy [13,14,15,16], have prompted the European LeukemiaNet to make several recommendations regarding MRD testing. These recommendations include (1) using MRD status as a factor in patient response criteria, (2) employing MRD status to drive post-remission therapy decisions and emphasizing the achievement of MRD-negative morphologic complete remission as a purpose of treatment, and (3) the utilization of MRD negativity as an end point in clinical trials as a surrogate marker for event-free survival [17].
Two primary methods exist for MRD testing: multiparameter flow cytometry (MFC) to assess for immunophenotypic abnormalities on blasts and molecular methods to assess for leukemia-associated genetic alterations. MRD status may be assessed either by MFC using a leukemia-associated immunophenotype approach that looks for a constellation of phenotypic markers previously seen at diagnosis in a specific patient or by a different-from-normal approach that looks for a discrete blast population with phenotypic characteristics unlike those of normal myeloblasts. Current consensus MFC MRD recommendations suggest using a combination of these strategies [12]. While MFC approaches can be attempted across AML subtypes (though with potentially less success in AML with certain phenotypes, such as those with monocytic differentiation [18]), molecular approaches must be tailored to the individual patient, and the wide availability of genetic panels designed for AML at diagnosis can lead to confusion among pathologists and clinicians regarding appropriate ordering of follow-up molecular MRD studies. Some molecular strategies for detecting MRD, particularly those targeting NPM1 mutations and WHO entity-defining recurrent fusions (e.g., PML/RARA, RUNX1/RUNX1T1, and CBFB/MYH11), boast superior sensitivity when compared with MFC, but are limited in applicability due to the specificity of their genetic targets. Exciting new developments in MRD assessment by next-generation sequencing (NGS) technologies may offer a broader, more flexible evaluation for persistent disease, but are currently limited by subpar sensitivity and a lack of clarity regarding which molecular aberrations are tied to specific clinical outcomes in specific subsets of patients. As massively parallel sequencing becomes more commonplace and the significance of specific mutations becomes better understood, new advancements in MRD detection and clinical utilization are likely to follow. In this review, we touch on the history of using molecular methods in AML MRD detection, discuss current molecular approaches, examine the most recent recommendations for molecular MRD use and the application of this data in clinical decision-making, and what the future may hold for MRD as new technologies come into the fold.
Quantitative PCR–based methods
PCR-based methods were first proposed for the detection of MRD in ALL and CML in the late 1980s [19, 20] and continue to serve as a critical component of disease monitoring in these patients [8, 10]. Currently, approximately 60% of AML cases in younger adults (under age 60) carry a recurrent molecular lesion that can be monitored post-therapy by widely validated quantitative reverse-transcriptase polymerase chain reaction (RT-qPCR) assays [17]. As a result, current ELN MRD Working Party consensus guidelines recommend this method for MRD monitoring in patients with acute promyelocytic leukemia (APL), core binding factor (CBF) AML, and AML with NPM1 mutations [12]. While bolstered by strong data that these lesions are highly stable through relapse, each of these molecular targets warrants some special considerations for optimal utilization. A number of other potential MRD targets can theoretically be assayed by qPCR in appropriate patient populations; these will also be briefly discussed.
Leukemia-associated fusions
RT-PCR methods to identify chimeric, leukemia-associated gene fusions are technically straightforward (Fig. 1a). Briefly, reverse transcription is performed on extracted RNA to generate complementary DNA (cDNA). While the genomic DNA intronic breakpoints may be highly variable in recurrently translocated genes, generally only a limited number of exons will combine to form leukemogenic chimeric transcripts at the RNA level. Therefore, relatively few primers are required to detect chimeric fusions using a cDNA template. Because forward and reverse primers are only near one another when the target fusion is present, non-neoplastic cells will not generate a product during the PCR reaction, leading to very low limits of detection with this approach (around 10−5) [12].
Molecular approaches for measurable residual disease testing. a Leukemia-specific chimeric fusion transcripts are detected by reverse transcriptase polymerase chain reaction. Following reverse transcription of cDNA, primers (blue arrows) bind to the abnormal chimeric fusion transcript, leading to PCR amplification if it is present. In the absence of a fusion, the primers would bind many megabases apart or on different chromosomes, precluding amplification. This allows for very low limits of detection in specimens where a target fusion is present. b Mutation-specific primers can be designed for highly recurrent mutations and used in an allele-specific PCR reaction. In the example, a primer specific for an NPM1 insertion mutation allows amplification of mutated NPM1. However, the mutant-specific primer does not efficiently bind wild-type NPM1, again leading to very specific amplification and allowing for low limit of detection. c Next-generation sequencing approaches theoretically allow for broad identification of mutations, as the number of individual sequencing reads carrying a mutation can be quantified. However, sequencing reads contributed by non-neoplastic cells are also present in the analysis, along with relatively high background sequencing error rates, limiting the ability to reach very low limits of detection with conventional approaches
Acute promyelocytic leukemia
Modern therapy regimens have transformed APL from “the most malignant form of acute leukemia” [21] to a member of the favorable risk category, with recent clinical trials reporting long-term overall survival from 88 to 99% and incidence of relapse ranging from < 1 to 5% [22,23,24,25]. However, the minority of APL patients who experience recurrence are at risk for adverse outcomes related to relapse-induced coagulopathy and treatment-induced differentiation syndrome [26], and early therapeutic interventions based on post-remission MRD positivity have been shown to facilitate better outcomes when compared with treating patients at frank clinical relapse [14, 15, 27]. Because ATRA-based therapies drive promyelocyte maturation instead of directly facilitating blast death, some patients require up to 10 weeks after therapy to achieve morphologic remission [27]. Not surprisingly, PML/RARA positivity by RT-qPCR can also persist for some time after completing induction therapy without representing treatment failure or clinically relevant residual disease. Therefore, PML/RARA quantification is recommended in APL patients at the completion of consolidation to predict for relapse risk, and post-induction assessment of MRD status is not indicated [26, 27]. Current European APL-specific consensus guidelines suggest that bone marrow aspirate material is the optimal specimen for APL MRD testing [27], though National Comprehensive Cancer Network (NCCN) guidelines suggest using peripheral blood as the MRD specimen [28].
Historically, qualitative RT-PCR methods were used for MRD monitoring in APL patients; however, more recent guidelines suggest using RT-qPCR techniques, with MRD positivity being established when increasing PML/RARA transcripts are identified in 2 consecutive samples [27]. In APL patients who have achieved MRD negativity following consolidation, current guidelines only recommend additional serial monitoring at 3-month intervals for high-risk patients (based on presenting WBC counts > 10 × 109/L) [27].
Core binding factor Leukemias
AML cases with t(8;21), inv(16), and t(16;16) (CBF AMLs) are also highly amenable to MRD monitoring by RT-qPCR-based methods, with the greatest prognostic value after consolidation therapy [29,30,31]. Patients with CBFB/MYH11 fusions may particularly benefit from a molecular approach to MRD detection over MFC methods due to the myelomonocytic leukemia–associated immunophenotype of many of these cases, which may make blast identification by flow cytometric analysis difficult [32]. A prospective, non-randomized study was able to identify t(8;21) patients at high risk for relapse based on fusion transcript levels after second consolidation and demonstrated that these patients benefitted from allogeneic stem cell transplant when compared with additional chemotherapy, whereas low-risk patients had favorable outcomes with chemotherapy and autologous transplant [13].
Notably, using RT-qPCR as an MRD modality in CBF AML has two important limitations: (1) while very high and very low transcript levels strongly predict risk of relapse, intermediate levels inadequately stratify patients [32,33,34,35,36], and (2) a considerable number of patients in stable remission demonstrate persistent fusion transcript positivity [37,38,39,40]. These findings precluded the early widespread adoption of qualitative RT-qPCR approaches in CBF AML patients; however, subsequent studies established the value of RT-qPCR MRD monitoring with set fusion transcript level thresholds and with an emphasis on monitoring fusion transcript kinetics [29, 30, 33, 41, 42] in combination with multiparameter flow cytometry [32] for optimal prognostication. European LeukemiaNet guidelines recommend testing for CBF fusions in both blood and marrow specimens following induction therapy/prior to consolidation, at end of therapy/prior to allogeneic stem cell transplant, and at 3-month intervals for 2 years [12]. Of note, the interval between molecular relapse and morphologic recurrence is often very short in t(8;21) cases, prompting a suggestion that MRD status in the blood should be assessed at monthly intervals for at least the first year following end of treatment frequent intervals in these patients [42].
Acute myeloid leukemia with mutated NPM1
NPM1 frameshift mutations have been identified in a large percentage of AML patients with normal karyotype [43] and are associated with good prognosis in the absence of FLT3 internal tandem duplications (ITD) [44,45,46,47,48,49]. Following the mutation’s discovery, several studies evaluated NPM1 mutations as an MRD marker [50,51,52,53,54]. Although many of these early reports were able to predict impending hematologic relapse in patients based on suboptimal reduction in mutant transcripts after therapy or by detecting an increase in transcript levels on serial sampling, most were not adequately powered to demonstrate statistical differences in overall survival based on MRD status. More recent studies have been able to clearly demonstrate the value of NPM1-based mutational MRD testing in predicting risk of overt relapse and inferior patient outcomes [55, 56]. Most studies on NPM1 as an MRD marker have assessed the peripheral blood; either blood, marrow, or both have been suggested as an appropriate specimen types [12, 28]. As with CBF AML, NPM1 MRD studies are suggested after induction/prior to consolidation therapy, at end of therapy/prior to transplantation, and at 3-month intervals for 2 years [12].
The rationale for using NPM1 as an MRD target stems from several observations. Notably, NPM1 mutations are considerably more stable in relapse than are FLT3-ITDs, which are often also present in these patients [47, 50]. NPM1 mutant allele transcripts are highly expressed in leukemic cells, allowing modern RT-qPCR methods to reach sensitivities of 1 in 106 or 107 [17]. NPM1 is also appealing because almost all mutations are in exon 12, with approximately 90% limited to 3 specific mutations (types A, B, and D) [43, 55], making an allele-specific RT-qPCR detection strategy that captures the majority of mutations relatively straightforward (Fig. 1b). However, different NPM1 MRD assays offer differing degrees of coverage for specific NPM1 mutation types, and so determining that a NPM1 MRD assay will identify an individual patient’s NPM1 mutation is paramount before it is used for post-therapy monitoring. Identification of an NPM1 mutation through a traditional fragment-based assay is not sufficient for this determination, as most common NPM1 mutations (types A, B, C, D) all constitute 4 base pair insertions that appear identical upon fragment analysis. A reasonable approach would be to test a diagnostic specimen with the MRD assay of interest or by confirming the specific NPM1 mutation type through sequencing analysis. Given the frequent monocytic phenotype of NPM1-mutated AML, molecular MRD testing also has advantages over more conventional MFC-based MRD approaches (Fig. 2). The ability to identify patients with inferior outcome in this category of generally good prognosis AML aids in determining which subset of patients will benefit from additional intervention, which has been shown to improve patient outcomes whether prompting transplant [57, 58] or additional chemotherapy [16]. While the great majority of relapses in patients with NPM1-mutated AML also harbor NPM1 mutations, NPM1-wild-type relapses can occur, possibly stemming from a preleukemic clone of hematopoietic stem cells [59]. This finding suggests that MFC MRD approaches or broader molecular MRD approaches using NGS (discussed below) may have value in this group of patients, in addition to monitoring for NPM1 mutations.
Phenotypic measurable residual disease approaches may be limited in NPM1-mutated acute myeloid leukemia. a An example of an NPM1-mutated acute myeloid leukemia is shown, with numerous monoblasts and promonocytes (Wright-Giemsa, × 1000). However, flow cytometric analysis (b) demonstrates an expansion of CD34-negative cells with monocytic antigen expression (light blue) that are not reliably distinguished from monocytes by flow cytometry. This phenotype would be difficult to impossible to detect in the MRD setting by multiparameter flow cytometry, but quantitative NPM1 mutation testing would demonstrate the presence of persistent disease. c Representative image of quantitative NPM1 testing showing a positive result with a quantitative allele-specific RT-PCR assay. The positive NPM1 result is represented by the blue curve; an internal housekeeping gene control, ABL1, is represented by the green curve
Additional MRD targets amenable to qPCR
Many other genetic anomalies are recurrently noted in AML [60], and these lesions may also serve as potential candidates for MRD monitoring. Translocations involving KMT2A (also known as MLL) on chromosome 11q are common in ALL and can serve as targets in ALL MRD detection [61]. Although less common in AML, MRD assessment of KMT2A fusion transcripts has been described in several small studies and seems to have some empiric prognostic value, particularly for predicting long-term remission when transcript levels remain low [62,63,64,65]. Several other reports have also demonstrated validity in using KMT2A partial tandem duplications (PTD) [66, 67], t(6;9) DEK/NUP214 [68], t(1;22) RBM15/MRTFA [69, 70], and t(7;12) MNX1-ETV6 [71] as MRD strategies. Logically, targeting t(9;22) in cases of BCR/ABL1 positive AML (a provisional entity in the current WHO classification) [60] is also feasible as it is widely used in CML MRD assessment, but its clinical utility in this rare subtype of AML remains to be elucidated. While these targets are theoretically amenable to use as MRD markers, their scarcity and the complexity of clinical assay validation and maintenance have largely limited their availability as clinical tests.
Other theoretic targets, such as FLT3-ITD and CEBPA mutations, are not generally considered to be reliable MRD candidates. FLT3-ITDs commonly arise in a subclone of blasts, and while they are often present at relapse, FLT3-ITD-negative relapses sometimes occur in patients with AML that harbored FLT3-ITD at diagnosis [72, 73]. Additionally, conventional fragment analysis methods used to determine FLT3 status lack the analytic sensitivity generally required for MRD analysis. However, the identification of FLT3-ITDs following therapy may have prognostic value [58, 72], particularly in the era of therapeutic FLT3 inhibition, and informatics methods to more reliably identify FLT3-ITDs through NGS approaches have been developed [74]. CEBPA is a notoriously difficult gene to assess, as it is highly GC-rich and difficult to sequence by NGS techniques [75]. CEBPA mutations are relatively heterogeneous without hotspots, making development of targeted MRD assays difficult.
Next-generation sequencing
Current consensus guidelines do not include NGS as a tool for assessing MRD. However, NGS approaches are likely to become increasingly important in monitoring AML patients following therapy. NGS methods are able to provide real-time sequencing data on thousands upon thousands of DNA strands as they are extended base-by-base in a massively parallel fashion. The result is nucleotide-level sequencing data on numerous, overlapping DNA strands that, when oriented by computational informatics software, can be overlaid to determine the sequences of long stretches of DNA with far greater speed and efficiency than prior PCR methods or Sanger sequencing (Fig. 1c). An important aspect of NGS input is target enrichment, in which numerous copies of the same region of DNA are captured and amplified prior to sequencing. Parallel sequencing of many copies of the same DNA region allows for statistical assurance of the accuracy of the sequence and, in samples with sequence heterogeneity (e.g., samples containing a mixture of non-neoplastic cells and clonally related neoplastic cells), parallel sequencing facilitates the identification of mutations and the quantification of mutational burden expressed as a variant allele frequency (VAF).
The ability to assess the mutational status and VAF of a multitude of genes makes NGS a potentially valuable tool for MRD assessment in biologically diverse entities such as AML. NGS platforms could offer utility as an MRD platform for a substantially larger percentage of AML patients when compared with conventional qPCR methods. Data supporting the use of NGS panels as an MRD modality are sparse but encouraging thus far. Recent large-scale evaluations have demonstrated that 89–93% of AML patients have at least 1 mutation that can serve as a potential marker of residual disease and that persistence of NGS-detected mutations post-therapy is a poor prognostic indicator [76, 77]. Zhou et al. also demonstrated that patients meeting at least 1 criterion (pre-therapy mutations in CEBPA (monoallelic), CSF3R, or NRAS, or post-therapy MRD positivity on a 34-gene panel) had a higher risk of relapse and decreased overall survival and benefitted from transplant [78]. The results of an ongoing clinical trial (NCT02756962), designed to assess the value of treating AML patients based on NGS-derived MRD status, are expected to highlight the clinical utility of this approach.
However, the main limitation of NGS is its relatively poor limit of detection, with most current clinical platforms only reliably identifying variant alleles at frequencies of 1–2% [79], and many clinical laboratories institute even higher, more conservative thresholds for mutation calls, at around 5%. The background sequencing error rate is highly dependent upon the sequence context, and individual, variant-specific error profiles would need to be generated or error-correction approaches applied to drive the limit of detection lower [76, 80]. The limit of detection by NGS is also dependent on the nature of the mutation in question, as small insertion/deletion mutations could theoretically be identified at lower VAFs than are single nucleotide variants, due to the underlying error profiles of sequencing. As current guidelines for MRD negativity require an assay with a limit of detection of 0.1% or lower [12], conventional NGS approaches generally cannot meet this target. However, these limitations have not prevented some recent studies from demonstrating that MRD assessment by NGS has prognostic utility [76, 77], with the caveat that negativity using conventional NGS platforms should not be accepted as definitive evidence of MRD-negative status [81].
The significance of specific mutations as MRD markers in AML is an area of intense study that remains to be fully understood. This difficulty is at least in part due to heterogeneity in the mutational landscape among patients, making detailed statistical analyses difficult. Some investigators have partially circumvented this limitation by assessing the cumulative prognostic value of genes in shared pathways [77, 82] or by performing subanalyses after removing single or groups of genes from the statistical analysis [76, 77]. These strategies have shed some light on genes that may serve as reliable MRD candidates. Using this approach, and based on observations that these mutations are often cleared in patients that experience sustained remissions, FLT3 (both FLT3-ITD and FLT3 tyrosine kinase domain mutations), NRAS, KRAS, PTPN11, and KIT appear to be promising MRD candidates. Several small studies have also highlighted value in using IDH1/IDH2 mutations as MRD targets, either via NGS [83, 84] or digital droplet PCR [85].
The prognostic implications of other mutations, particularly founder-type mutations that are strongly associated with clonal hematopoiesis of indeterminate potential (CHIP), are more difficult to discern, a problem that was recently extensively reviewed by Hasserjian and colleagues [81]. Clones with mutations in DNMT3A, TET2, and ASXL1 (collectively referred to as “DTA mutations”) often persist or expand after AML-directed chemotherapy, as they are relatively chemoresistant [86]. The proposed relevance of DTA mutations in MRD assessment differs in various studies. Hirsch et al. noted that the persistence of CHIP lesions did not impact prognosis when assessed individually, but patients with ≥ 2 persistent mutations (presumably including CHIP lesions) did have poorer outcomes [82]. In contrast, several recent studies identified no prognostic value in persistent DTA mutations [76, 77]. Mutations in several non-DTA genes (e.g., BCOR and SRSF2) are also thought to be related to underlying CHIP, although these genes are less commonly encountered in AML and have not been assessed as rigorously [76, 81, 87].
Mutations in other genes, including TP53 and RUNX1, may have MRD utility in select patients but should be interpreted with caution. TP53 mutations are often a component of CHIP and thus lack clear-cut utility as an MRD marker [81]. However, excluding TP53 mutations from MRD statistical assessments weakened the prognostic value of mutation clearance in one analysis [77], and relapses appeared to occur in the majority of a small number of patients with persistent TP53 mutations post-therapy [82, 87]. Although RUNX1 mutations are a common finding in AML and define a new provisional diagnostic category in the current WHO classification [60], their utility as MRD targets may be limited due to their frequent presence in CHIP [81]. However, at least one study has demonstrated some value in detecting RUNX1 mutations in the setting of MRD [88]. Systematic implementation of NGS as an MRD strategy may be helpful in clarifying some of these areas of uncertainty, but it is clear that not all mutations have equivalent clinical implications for the patient, markedly complicating interpretation of MRD testing performed by NGS.
Future directions
Recent reports demonstrating improved analytic sensitivity of NGS-based methods by optimizing various aspects of workflow and informatics [89] or by utilizing massively multiplex digital PCR with primers that preferentially amplify variant alleles over wild type sequences [90] indicate that clinical NGS testing may someday boast sensitivities comparable to PCR-based approaches. NGS is particularly well-suited for monitoring AML cases that undergo clonal or immunophenotypic evolution after therapy or assessing for the emergence of unrelated, possibly therapy-induced, AML clones [91], a phenomenon that can undermine MFC- and qPCR-based MRD strategies [53, 92]. The finding of an unrelated AML clone may have implications for clinical trial eligibility, and with the emergence of more tailored AML therapies, repeated genetic characterization of AML at follow-up and relapse will continue to increase in importance [81]. Novel NGS strategies are also amenable to the identification of recurrent fusions and other large gene aberrations, such as tandem duplications, possibly allowing for NGS assessment of MRD to encompass biomarkers that have been traditionally evaluated by RT-qPCR [93, 94]. With continued technical advances, NGS platforms may allow molecular MRD testing to become closer to a one-size-fits-all approach, analogous to the current situation with MFC, rather than the more limited, individually tailored approach that is currently necessary and that is more likely to engender test ordering error and misinterpretation.
Conclusions and take-home points
MRD detection in AML is a challenging and dynamic endeavor, due to both advancements in our understanding of AML biology and new technological approaches. MFC modalities have typically been favored over PCR-based approaches due to their applicability in a greater percentage of patients. However, MFC assays are difficult to standardize and may be problematic for cases with certain phenotypes (e.g., myelomonocytic cases) and cases with immunophenotypic shift secondary to clonal evolution. Current guidelines emphasize that molecular MRD studies are indicated for patients whose AMLs harbor PML/RARA, RUNX1/RUNX1T1, CBFB/MYH11, or NPM1 mutations, while MFC should serve as the primary MRD method for other AML subtypes [12]. For AML patients with less commonly encountered stable fusions, qPCR could also serve an empiric role in disease monitoring, but lack of widespread assay availability is a major limiting factor.
Newer NGS approaches are promising but are partially compromised by suboptimal analytic sensitivity and an incomplete understanding of the importance of specific mutations in the post-therapy setting. However, recent improvements in assay workflow and informatic processing may close the gap in sensitivity between NGS and qPCR. Even with a relatively poor limit of detection, several studies highlight the prognostic value of NGS as an MRD strategy, and an ongoing clinical trial will likely further strengthen our understanding of how MRD information can best be used to improve patient outcomes. MFC and MRD testing by NGS are orthogonal approaches that may best be used in parallel, as the combined data may best identify patients at risk for relapse [76]. A current practical limiting factor for the uptake of NGS MRD methods in AML relates to issues surrounding test reimbursement, as these studies may not currently be covered by payors despite the emerging data regarding clinical relevance [95].
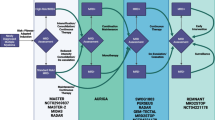
MRD studies in AML patients are now suggested as part of the standard follow-up of the disease (Table 1). Molecular MRD studies are generally indicated following induction/prior to consolidation, at the end of treatment/prior to bone marrow transplant, and at 3-month intervals following end of therapy for patients with CBF AML, and AML with mutated NPM1, while APL patients should be monitored after consolidation and at 3-month intervals in high-risk patients (those with initial WBC counts > 10 × 109/L) [12, 27]. Other patients should be followed by MFC MRD studies; however, incorporation of NGS studies may also be reasonable, with the caveat that NGS negativity is not currently equivalent to MRD negativity, due to the suboptimal limit of detection of NGS.
As follow-up testing for AML patients is complex and tailored to the patient’s initial disease genetics, institutions should consider developing standardized workflows and procedures to facilitate testing and to avoid inappropriate test usage. Quantitative molecular MRD studies require RNA as a template, and so laboratories should establish procedures to extract both DNA and RNA from bone marrow specimens from patients with AML. Hematopathologists should consider reaching consensus with local molecular pathology and clinical colleagues to determine the appropriate use of available myeloid NGS testing in the follow-up setting, and pathologists should remain abreast of changing guidelines and technologies in this rapidly evolving field.
References
Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Löwenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD (2003) Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 21(24):4642–4649. https://doi.org/10.1200/JCO.2003.04.036
Cheson BD, Cassileth PA, Head DR, Schiffer CA, Bennett JM, Bloomfield CD, Brunning R, Gale RP, Grever MR, Keating MJ (1990) Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol 8(5):813–819. https://doi.org/10.1200/JCO.1990.8.5.813
Inaba H, Coustan-Smith E, Cao X, Pounds SB, Shurtleff SA, Wang KY, Raimondi SC, Onciu M, Jacobsen J, Ribeiro RC, Dahl GV, Bowman WP, Taub JW, Degar B, Leung W, Downing JR, Pui C-H, Rubnitz JE, Campana D (2012) Comparative analysis of different approaches to measure treatment response in acute myeloid leukemia. J Clin Oncol 30(29):3625–3632. https://doi.org/10.1200/JCO.2011.41.5323
Loken MR, Alonzo TA, Pardo L, Gerbing RB, Raimondi SC, Hirsch BA, Ho PA, Franklin J, Cooper TM, Gamis AS, Meshinchi S (2012) Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: a report from Children’s Oncology Group. Blood 120(8):1581–1588. https://doi.org/10.1182/blood-2012-02-408336
Vollmer RT (2009) Blast counts in bone marrow aspirate smears: analysis using the Poisson probability function, Bayes theorem, and information theory. Am J Clin Pathol 131(2):183–188. https://doi.org/10.1309/ajcpbayncu35zgzg
Harrison WJ (1962) The total cellularity of the bone marrow in man. J Clin Pathol 15(3):254. https://doi.org/10.1136/jcp.15.3.254
Hourigan CS, Gale RP, Gormley NJ, Ossenkoppele GJ, Walter RB (2017) Measurable residual disease testing in acute myeloid leukaemia. Leukemia 31(7):1482–1490. https://doi.org/10.1038/leu.2017.113
Berry DA, Zhou S, Higley H, Mukundan L, Fu S, Reaman GH, Wood BL, Kelloff GJ, Jessup JM, Radich JP (2017) Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol 3(7):e170580. https://doi.org/10.1001/jamaoncol.2017.0580
Beldjord K, Chevret S, Asnafi V, Huguet F, Boulland ML, Leguay T, Thomas X, Cayuela JM, Grardel N, Chalandon Y, Boissel N, Schaefer B, Delabesse E, Cave H, Chevallier P, Buzyn A, Fest T, Reman O, Vernant JP, Lheritier V, Bene MC, Lafage M, Macintyre E, Ifrah N, Dombret H, Group for Research on Adult Acute Lymphoblastic L (2014) Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood 123(24):3739–3749. https://doi.org/10.1182/blood-2014-01-547695
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, Cervantes F, Clark RE, Cortes JE, Guilhot F, Hjorth-Hansen H, Hughes TP, Kantarjian HM, Kim DW, Larson RA, Lipton JH, Mahon FX, Martinelli G, Mayer J, Muller MC, Niederwieser D, Pane F, Radich JP, Rousselot P, Saglio G, Saussele S, Schiffer C, Silver R, Simonsson B, Steegmann JL, Goldman JM, Hehlmann R (2013) European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood 122(6):872–884. https://doi.org/10.1182/blood-2013-05-501569
Grimwade D, Freeman SD (2014) Defining minimal residual disease in acute myeloid leukemia: which platforms are ready for “prime time”? Blood 124(23):3345–3355. https://doi.org/10.1182/blood-2014-05-577593
Schuurhuis GJ, Heuser M, Freeman S, Bene MC, Buccisano F, Cloos J, Grimwade D, Haferlach T, Hills RK, Hourigan CS, Jorgensen JL, Kern W, Lacombe F, Maurillo L, Preudhomme C, van der Reijden BA, Thiede C, Venditti A, Vyas P, Wood BL, Walter RB, Dohner K, Roboz GJ, Ossenkoppele GJ (2018) Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood 131(12):1275–1291. https://doi.org/10.1182/blood-2017-09-801498
Zhu HH, Zhang XH, Qin YZ, Liu DH, Jiang H, Chen H, Jiang Q, Xu LP, Lu J, Han W, Bao L, Wang Y, Chen YH, Wang JZ, Wang FR, Lai YY, Chai JY, Wang LR, Liu YR, Liu KY, Jiang B, Huang XJ (2013) MRD-directed risk stratification treatment may improve outcomes of t(8;21) AML in the first complete remission: results from the AML05 multicenter trial. Blood 121(20):4056–4062. https://doi.org/10.1182/blood-2012-11-468348
Lo Coco F, Diverio D, Avvisati G, Petti MC, Meloni G, Pogliani EM, Biondi A, Rossi G, Carlo-Stella C, Selleri C, Martino B, Specchia G, Mandelli F (1999) Therapy of molecular relapse in acute promyelocytic leukemia. Blood 94(7):2225–2229
Esteve J, Escoda L, Martin G, Rubio V, Diaz-Mediavilla J, Gonzalez M, Rivas C, Alvarez C, Gonzalez San Miguel JD, Brunet S, Tomas JF, Tormo M, Sayas MJ, Sanchez Godoy P, Colomer D, Bolufer P, Sanz MA, Spanish Cooperative Group P (2007) Outcome of patients with acute promyelocytic leukemia failing to front-line treatment with all-trans retinoic acid and anthracycline-based chemotherapy (PETHEMA protocols LPA96 and LPA99): benefit of an early intervention. Leukemia 21(3):446–452. https://doi.org/10.1038/sj.leu.2404501
Platzbecker U, Middeke JM, Sockel K, Herbst R, Wolf D, Baldus CD, Oelschlagel U, Mutherig A, Fransecky L, Noppeney R, Bug G, Gotze KS, Kramer A, Bochtler T, Stelljes M, Groth C, Schubert A, Mende M, Stolzel F, Borkmann C, Kubasch AS, von Bonin M, Serve H, Hanel M, Duhrsen U, Schetelig J, Rollig C, Kramer M, Ehninger G, Bornhauser M, Thiede C (2018) Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA2): an open-label, multicentre, phase 2 trial. Lancet Oncol 19(12):1668–1679. https://doi.org/10.1016/S1470-2045(18)30580-1
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Lowenberg B, Bloomfield CD (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129(4):424–447. https://doi.org/10.1182/blood-2016-08-733196
Xu J, Jorgensen JL, Wang SA (2017) How do we use multicolor flow cytometry to detect minimal residual disease in acute myeloid leukemia? Clin Lab Med 37(4):787–802. https://doi.org/10.1016/j.cll.2017.07.004
Wright JJ, Poplack DG, Bakhshi A, Reaman G, Cole D, Jensen JP, Korsmeyer SJ (1987) Gene rearrangements as markers of clonal variation and minimal residual disease in acute lymphoblastic leukemia. J Clin Oncol 5(5):735–741. https://doi.org/10.1200/JCO.1987.5.5.735
Lee MS, Chang KS, Freireich EJ, Kantarjian HM, Talpaz M, Trujillo JM, Stass SA (1988) Detection of minimal residual bcr/abl transcripts by a modified polymerase chain reaction. Blood 72(3):893–897
Hillestad LK (1957) Acute promyelocytic leukemia. Acta Med Scand 159(3):189–194
Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, Ferrara F, Fazi P, Cicconi L, Di Bona E, Specchia G, Sica S, Divona M, Levis A, Fiedler W, Cerqui E, Breccia M, Fioritoni G, Salih HR, Cazzola M, Melillo L, Carella AM, Brandts CH, Morra E, von Lilienfeld-Toal M, Hertenstein B, Wattad M, Lubbert M, Hanel M, Schmitz N, Link H, Kropp MG, Rambaldi A, La Nasa G, Luppi M, Ciceri F, Finizio O, Venditti A, Fabbiano F, Dohner K, Sauer M, Ganser A, Amadori S, Mandelli F, Dohner H, Ehninger G, Schlenk RF, Platzbecker U, Gruppo Italiano Malattie Ematologiche dA, German-Austrian Acute Myeloid Leukemia Study G, Study Alliance L (2013) Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med 369(2):111–121. https://doi.org/10.1056/NEJMoa1300874
Burnett AK, Russell NH, Hills RK, Bowen D, Kell J, Knapper S, Morgan YG, Lok J, Grech A, Jones G, Khwaja A, Friis L, McMullin MF, Hunter A, Clark RE, Grimwade D, Group UKNCRIAMLW (2015) Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol 16(13):1295–1305. https://doi.org/10.1016/S1470-2045(15)00193-X
Iland HJ, Collins M, Bradstock K, Supple SG, Catalano A, Hertzberg M, Browett P, Grigg A, Firkin F, Campbell LJ, Hugman A, Reynolds J, Di Iulio J, Tiley C, Taylor K, Filshie R, Seldon M, Taper J, Szer J, Moore J, Bashford J, Seymour JF, Australasian L, Lymphoma G (2015) Use of arsenic trioxide in remission induction and consolidation therapy for acute promyelocytic leukaemia in the Australasian Leukaemia and Lymphoma Group (ALLG) APML4 study: a non-randomised phase 2 trial. Lancet Haematol 2(9):e357–e366. https://doi.org/10.1016/S2352-3026(15)00115-5
Abaza Y, Kantarjian H, Garcia-Manero G, Estey E, Borthakur G, Jabbour E, Faderl S, O'Brien S, Wierda W, Pierce S, Brandt M, McCue D, Luthra R, Patel K, Kornblau S, Kadia T, Daver N, DiNardo C, Jain N, Verstovsek S, Ferrajoli A, Andreeff M, Konopleva M, Estrov Z, Foudray M, McCue D, Cortes J, Ravandi F (2017) Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood 129(10):1275–1283. https://doi.org/10.1182/blood-2016-09-736686
Grimwade D, Jovanovic JV, Hills RK, Nugent EA, Patel Y, Flora R, Diverio D, Jones K, Aslett H, Batson E, Rennie K, Angell R, Clark RE, Solomon E, Lo-Coco F, Wheatley K, Burnett AK (2009) Prospective minimal residual disease monitoring to predict relapse of acute promyelocytic leukemia and to direct pre-emptive arsenic trioxide therapy. J Clin Oncol 27(22):3650–3658. https://doi.org/10.1200/JCO.2008.20.1533
Sanz MA, Fenaux P, Tallman MS, Estey EH, Lowenberg B, Naoe T, Lengfelder E, Dohner H, Burnett AK, Chen SJ, Mathews V, Iland H, Rego E, Kantarjian H, Ades L, Avvisati G, Montesinos P, Platzbecker U, Ravandi F, Russell NH, Lo-Coco F (2019) Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood 133(15):1630–1643. https://doi.org/10.1182/blood-2019-01-894980
Network NCC Acute Myeloid Leukemia (Version 2.2021). https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf. Accessed December 11, 2020
Agrawal M, Corbacioglu A, Paschka P, Weber D, Gaidzik VI, Jahn N, Kündgen A, Kindler T, Wattad MA, Lübbert M, Fiedler W, Götze K, Ringhoffer M, Schleicher J, Lange E, Held G, Griesshammer M, Greil R, Kirchen H, Koller E, Thol F, Krauter J, Heuser M, Ganser A, Bullinger L, Schlenk RF, Döhner H, Döhner K (2016) Minimal residual disease monitoring in acute myeloid leukemia (AML) with translocation t(8;21)(q22;q22): results of the AML Study Group (AMLSG). Blood 128(22):1207–1207. https://doi.org/10.1182/blood.V128.22.1207.1207
Jourdan E, Boissel N, Chevret S, Delabesse E, Renneville A, Cornillet P, Blanchet O, Cayuela JM, Recher C, Raffoux E, Delaunay J, Pigneux A, Bulabois CE, Berthon C, Pautas C, Vey N, Lioure B, Thomas X, Luquet I, Terre C, Guardiola P, Bene MC, Preudhomme C, Ifrah N, Dombret H, French AMLI (2013) Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood 121(12):2213–2223. https://doi.org/10.1182/blood-2012-10-462879
Weisser M, Haferlach C, Hiddemann W, Schnittger S (2007) The quality of molecular response to chemotherapy is predictive for the outcome of AML1-ETO-positive AML and is independent of pretreatment risk factors. Leukemia 21(6):1177–1182. https://doi.org/10.1038/sj.leu.2404659
Ouyang J, Goswami M, Peng J, Zuo Z, Daver N, Borthakur G, Tang G, Medeiros LJ, Jorgensen JL, Ravandi F, Wang SA (2016) Comparison of multiparameter flow cytometry immunophenotypic analysis and quantitative RT-PCR for the detection of minimal residual disease of core binding factor acute myeloid leukemia. Am J Clin Pathol 145(6):769–777. https://doi.org/10.1093/ajcp/aqw038
Yin JA, O'Brien MA, Hills RK, Daly SB, Wheatley K, Burnett AK (2012) Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: results of the United Kingdom MRC AML-15 trial. Blood 120(14):2826–2835. https://doi.org/10.1182/blood-2012-06-435669
Krauter J, Gorlich K, Ottmann O, Lubbert M, Dohner H, Heit W, Kanz L, Ganser A, Heil G (2003) Prognostic value of minimal residual disease quantification by real-time reverse transcriptase polymerase chain reaction in patients with core binding factor leukemias. J Clin Oncol 21(23):4413–4422. https://doi.org/10.1200/JCO.2003.03.166
Morschhauser F, Cayuela JM, Martini S, Baruchel A, Rousselot P, Socie G, Berthou P, Jouet JP, Straetmans N, Sigaux F, Fenaux P, Preudhomme C (2000) Evaluation of minimal residual disease using reverse-transcription polymerase chain reaction in t(8;21) acute myeloid leukemia: a multicenter study of 51 patients. J Clin Oncol 18(4):788–794. https://doi.org/10.1200/JCO.2000.18.4.788
van der Reijden BA, Simons A, Luiten E, van der Poel SC, Hogenbirk PE, Tonnissen E, Valk PJ, Lowenberg B, De Greef GE, Breuning MH, Jansen JH (2002) Minimal residual disease quantification in patients with acute myeloid leukaemia and inv(16)/CBFB-MYH11 gene fusion. Br J Haematol 118(2):411–418. https://doi.org/10.1046/j.1365-2141.2002.03738.x
Nucifora G, Larson RA, Rowley JD (1993) Persistence of the 8;21 translocation in patients with acute myeloid leukemia type M2 in long-term remission. Blood 82(3):712–715
Kusec R, Laczika K, Knobl P, Friedl J, Greinix H, Kahls P, Linkesch W, Schwarzinger I, Mitterbauer G, Purtscher B et al (1994) AML1/ETO fusion mRNA can be detected in remission blood samples of all patients with t(8;21) acute myeloid leukemia after chemotherapy or autologous bone marrow transplantation. Leukemia 8(5):735–739
Tobal K, Johnson PR, Saunders MJ, Harrison CJ, Liu Yin JA (1995) Detection of CBFB/MYH11 transcripts in patients with inversion and other abnormalities of chromosome 16 at presentation and remission. Br J Haematol 91(1):104–108. https://doi.org/10.1111/j.1365-2141.1995.tb05253.x
Elmaagacli AH, Beelen DW, Kroll M, Trzensky S, Stein C, Schaefer UW (1998) Detection of CBFbeta/MYH11 fusion transcripts in patients with inv(16) acute myeloid leukemia after allogeneic bone marrow or peripheral blood progenitor cell transplantation. Bone Marrow Transplant 21(2):159–166. https://doi.org/10.1038/sj.bmt.1701056
Willekens C, Blanchet O, Renneville A, Cornillet-Lefebvre P, Pautas C, Guieze R, Ifrah N, Dombret H, Jourdan E, Preudhomme C, Boissel N, French AMLI (2016) Prospective long-term minimal residual disease monitoring using RQ-PCR in RUNX1-RUNX1T1-positive acute myeloid leukemia: results of the French CBF-2006 trial. Haematologica 101(3):328–335. https://doi.org/10.3324/haematol.2015.131946
Rucker FG, Agrawal M, Corbacioglu A, Weber D, Kapp-Schwoerer S, Gaidzik VI, Jahn N, Schroeder T, Wattad M, Lubbert M, Koller E, Kindler T, Gotze K, Ringhoffer M, Westermann J, Fiedler W, Horst HA, Greil R, Schroers R, Mayer K, Heinicke T, Krauter J, Schlenk RF, Thol F, Heuser M, Ganser A, Bullinger L, Paschka P, Dohner H, Dohner K (2019) Measurable residual disease monitoring in acute myeloid leukemia with t(8;21)(q22;q22.1): results from the AML Study Group. Blood 134(19):1608–1618. https://doi.org/10.1182/blood.2019001425
Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, La Starza R, Diverio D, Colombo E, Santucci A, Bigerna B, Pacini R, Pucciarini A, Liso A, Vignetti M, Fazi P, Meani N, Pettirossi V, Saglio G, Mandelli F, Lo-Coco F, Pelicci PG, Martelli MF, Party GALW (2005) Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med 352(3):254–266. https://doi.org/10.1056/NEJMoa041974
Cazzaniga G, Dell'Oro MG, Mecucci C, Giarin E, Masetti R, Rossi V, Locatelli F, Martelli MF, Basso G, Pession A, Biondi A, Falini B (2005) Nucleophosmin mutations in childhood acute myelogenous leukemia with normal karyotype. Blood 106(4):1419–1422. https://doi.org/10.1182/blood-2005-03-0899
Noguera NI, Ammatuna E, Zangrilli D, Lavorgna S, Divona M, Buccisano F, Amadori S, Mecucci C, Falini B, Lo-Coco F (2005) Simultaneous detection of NPM1 and FLT3-ITD mutations by capillary electrophoresis in acute myeloid leukemia. Leukemia 19(8):1479–1482. https://doi.org/10.1038/sj.leu.2403846
Schnittger S, Schoch C, Kern W, Mecucci C, Tschulik C, Martelli MF, Haferlach T, Hiddemann W, Falini B (2005) Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood 106(12):3733–3739. https://doi.org/10.1182/blood-2005-06-2248
Suzuki T, Kiyoi H, Ozeki K, Tomita A, Yamaji S, Suzuki R, Kodera Y, Miyawaki S, Asou N, Kuriyama K, Yagasaki F, Shimazaki C, Akiyama H, Nishimura M, Motoji T, Shinagawa K, Takeshita A, Ueda R, Kinoshita T, Emi N, Naoe T (2005) Clinical characteristics and prognostic implications of NPM1 mutations in acute myeloid leukemia. Blood 106(8):2854–2861. https://doi.org/10.1182/blood-2005-04-1733
Dohner K, Schlenk RF, Habdank M, Scholl C, Rucker FG, Corbacioglu A, Bullinger L, Frohling S, Dohner H (2005) Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood 106(12):3740–3746. https://doi.org/10.1182/blood-2005-05-2164
Verhaak RG, Goudswaard CS, van Putten W, Bijl MA, Sanders MA, Hugens W, Uitterlinden AG, Erpelinck CA, Delwel R, Lowenberg B, Valk PJ (2005) Mutations in nucleophosmin (NPM1) in acute myeloid leukemia (AML): association with other gene abnormalities and previously established gene expression signatures and their favorable prognostic significance. Blood 106(12):3747–3754. https://doi.org/10.1182/blood-2005-05-2168
Chou WC, Tang JL, Wu SJ, Tsay W, Yao M, Huang SY, Huang KC, Chen CY, Huang CF, Tien HF (2007) Clinical implications of minimal residual disease monitoring by quantitative polymerase chain reaction in acute myeloid leukemia patients bearing nucleophosmin (NPM1) mutations. Leukemia 21(5):998–1004. https://doi.org/10.1038/sj.leu.2404637
Dvorakova D, Lengerova M, Pospisilova J, Palasek I, Mayer J (2009) A novel quantitative assessment of minimal residual disease in patients with acute myeloid leukemia carrying NPM1 (nucleophosmin) exon 12 mutations. Leukemia 23(4):793–796. https://doi.org/10.1038/leu.2008.268
Gorello P, Cazzaniga G, Alberti F, Dell'Oro MG, Gottardi E, Specchia G, Roti G, Rosati R, Martelli MF, Diverio D, Lo Coco F, Biondi A, Saglio G, Mecucci C, Falini B (2006) Quantitative assessment of minimal residual disease in acute myeloid leukemia carrying nucleophosmin (NPM1) gene mutations. Leukemia 20(6):1103–1108. https://doi.org/10.1038/sj.leu.2404149
Papadaki C, Dufour A, Seibl M, Schneider S, Bohlander SK, Zellmeier E, Mellert G, Hiddemann W, Spiekermann K (2009) Monitoring minimal residual disease in acute myeloid leukaemia with NPM1 mutations by quantitative PCR: clonal evolution is a limiting factor. Br J Haematol 144(4):517–523. https://doi.org/10.1111/j.1365-2141.2008.07488.x
Schnittger S, Kern W, Tschulik C, Weiss T, Dicker F, Falini B, Haferlach C, Haferlach T (2009) Minimal residual disease levels assessed by NPM1 mutation-specific RQ-PCR provide important prognostic information in AML. Blood 114(11):2220–2231. https://doi.org/10.1182/blood-2009-03-213389
Ivey A, Hills RK, Simpson MA, Jovanovic JV, Gilkes A, Grech A, Patel Y, Bhudia N, Farah H, Mason J, Wall K, Akiki S, Griffiths M, Solomon E, McCaughan F, Linch DC, Gale RE, Vyas P, Freeman SD, Russell N, Burnett AK, Grimwade D, Group UKNCRIAW (2016) Assessment of minimal residual disease in standard-risk AML. N Engl J Med 374(5):422–433. https://doi.org/10.1056/NEJMoa1507471
Kronke J, Schlenk RF, Jensen KO, Tschurtz F, Corbacioglu A, Gaidzik VI, Paschka P, Onken S, Eiwen K, Habdank M, Spath D, Lubbert M, Wattad M, Kindler T, Salih HR, Held G, Nachbaur D, von Lilienfeld-Toal M, Germing U, Haase D, Mergenthaler HG, Krauter J, Ganser A, Gohring G, Schlegelberger B, Dohner H, Dohner K (2011) Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol 29(19):2709–2716. https://doi.org/10.1200/JCO.2011.35.0371
Balsat M, Renneville A, Thomas X, de Botton S, Caillot D, Marceau A, Lemasle E, Marolleau JP, Nibourel O, Berthon C, Raffoux E, Pigneux A, Rodriguez C, Vey N, Cayuela JM, Hayette S, Braun T, Coude MM, Terre C, Celli-Lebras K, Dombret H, Preudhomme C, Boissel N (2017) Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: a study by the Acute Leukemia French Association Group. J Clin Oncol 35(2):185–193. https://doi.org/10.1200/JCO.2016.67.1875
Dillon R, Hills R, Freeman S, Potter N, Jovanovic J, Ivey A, Kanda AS, Runglall M, Foot N, Valganon M, Khwaja A, Cavenagh J, Smith M, Ommen HB, Overgaard UM, Dennis M, Knapper S, Kaur H, Taussig D, Mehta P, Raj K, Novitzky-Basso I, Nikolousis E, Danby R, Krishnamurthy P, Hill K, Finnegan D, Alimam S, Hurst E, Johnson P, Khan A, Salim R, Craddock C, Spearing R, Gilkes A, Gale R, Burnett A, Russell NH, Grimwade D, Group obotUNCRIAMLW (2020) Molecular MRD status and outcome after transplantation in NPM1-mutated AML. Blood 135(9):680–688. https://doi.org/10.1182/blood.2019002959
Höllein A, Meggendorfer M, Dicker F, Jeromin S, Nadarajah N, Kern W, Haferlach C, Haferlach T (2018) NPM1 mutated AML can relapse with wild-type NPM1: persistent clonal hematopoiesis can drive relapse. Blood Adv 2(22):3118–3125. https://doi.org/10.1182/bloodadvances.2018023432
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J (eds) (2017) WHO classification of tumours of haematopoietic and lymphoid tissues, Revised 4th ed. IARC, Lyon
Burmeister T, Marschalek R, Schneider B, Meyer C, Gokbuget N, Schwartz S, Hoelzer D, Thiel E (2006) Monitoring minimal residual disease by quantification of genomic chromosomal breakpoint sequences in acute leukemias with MLL aberrations. Leukemia 20(3):451–457. https://doi.org/10.1038/sj.leu.2404082
Huang S, Yang H, Li Y, Feng C, Gao L, Chen GF, Gao HH, Huang Z, Li YH, Yu L (2016) Prognostic Significance of Mixed-Lineage Leukemia (MLL) Gene detected by real-time fluorescence quantitative PCR assay in acute myeloid leukemia. Med Sci Monit 22:3009–3017. https://doi.org/10.12659/msm.900429
Matsuo H, Iijima-Yamashita Y, Yamada M, Deguchi T, Kiyokawa N, Shimada A, Tawa A, Tomizawa D, Taga T, Kinoshita A, Adachi S, Horibe K (2018) Monitoring of fusion gene transcripts to predict relapse in pediatric acute myeloid leukemia. Pediatr Int 60(1):41–46. https://doi.org/10.1111/ped.13440
Scholl C, Schlenk RF, Eiwen K, Dohner H, Frohling S, Dohner K, Group AMLS (2005) The prognostic value of MLL-AF9 detection in patients with t(9;11)(p22;q23)-positive acute myeloid leukemia. Haematologica 90(12):1626–1634
Mitterbauer G, Zimmer C, Pirc-Danoewinata H, Haas OA, Hojas S, Schwarzinger I, Greinix H, Jager U, Lechner K, Mannhalter C (2000) Monitoring of minimal residual disease in patients with MLL-AF6-positive acute myeloid leukaemia by reverse transcriptase polymerase chain reaction. Br J Haematol 109(3):622–628. https://doi.org/10.1046/j.1365-2141.2000.02076.x
Weisser M, Kern W, Schoch C, Hiddemann W, Haferlach T, Schnittger S (2005) Risk assessment by monitoring expression levels of partial tandem duplications in the MLL gene in acute myeloid leukemia during therapy. Haematologica 90(7):881–889
Ommen HB, Hokland P, Haferlach T, Abildgaard L, Alpermann T, Haferlach C, Kern W, Schnittger S (2014) Relapse kinetics in acute myeloid leukaemias with MLL translocations or partial tandem duplications within the MLL gene. Br J Haematol 165(5):618–628. https://doi.org/10.1111/bjh.12792
Ostergaard M, Stentoft J, Hokland P (2004) A real-time quantitative RT-PCR assay for monitoring DEK-CAN fusion transcripts arising from translocation t(6;9) in acute myeloid leukemia. Leuk Res 28(11):1213–1215. https://doi.org/10.1016/j.leukres.2004.03.011
Ballerini P, Blaise A, Mercher T, Pellegrino B, Perot C, van den Akker J, Gatbois E, Adam M, Douay L, Berger R, Bernard O, Landman-Parker J (2003) A novel real-time RT-PCR assay for quantification of OTT-MAL fusion transcript reliable for diagnosis of t(1;22) and minimal residual disease (MRD) detection. Leukemia 17(6):1193–1196. https://doi.org/10.1038/sj.leu.2402914
Takeda A, Shimada A, Hamamoto K, Yoshino S, Nagai T, Fujii Y, Yamada M, Nakamura Y, Watanabe T, Watanabe Y, Yamamoto Y, Sakakibara K, Oda M, Morishima T (2014) Detection of RBM15-MKL1 fusion was useful for diagnosis and monitoring of minimal residual disease in infant acute megakaryoblastic leukemia. Acta Med Okayama 68(2):119–123. https://doi.org/10.18926/AMO/52408
Hauer J, Tosi S, Schuster FR, Harbott J, Kolb HJ, Borkhardt A (2008) Graft versus leukemia effect after haploidentical HSCT in a MLL-negative infant AML with HLXB9/ETV6 rearrangement. Pediatr Blood Cancer 50(4):921–923. https://doi.org/10.1002/pbc.21376
Abdelhamid E, Preudhomme C, Helevaut N, Nibourel O, Gardin C, Rousselot P, Castaigne S, Gruson B, Berthon C, Soua Z, Renneville A (2012) Minimal residual disease monitoring based on FLT3 internal tandem duplication in adult acute myeloid leukemia. Leuk Res 36(3):316–323. https://doi.org/10.1016/j.leukres.2011.11.002
Shih L-Y, Huang C-F, Wu J-H, Lin T-L, Dunn P, Wang P-N, Kuo M-C, Lai C-L, Hsu H-C (2002) Internal tandem duplication of FLT3 in relapsed acute myeloid leukemia: a comparative analysis of bone marrow samples from 108 adult patients at diagnosis and relapse. Blood 100(7):2387–2392. https://doi.org/10.1182/blood-2002-01-0195
Blätte TJ, Schmalbrock LK, Skambraks S, Lux S, Cocciardi S, Dolnik A, Döhner H, Döhner K, Bullinger L (2019) getITD for FLT3-ITD-based MRD monitoring in AML. Leukemia 33(10):2535–2539. https://doi.org/10.1038/s41375-019-0483-z
Behdad A, Weigelin HC, Elenitoba-Johnson KSJ, Betz BL (2015) A clinical grade sequencing-based assay for CEBPA mutation testing: report of a large series of myeloid neoplasms. J Mol Diagn 17(1):76–84. https://doi.org/10.1016/j.jmoldx.2014.09.007
Jongen-Lavrencic M, Grob T, Hanekamp D, Kavelaars FG, Al Hinai A, Zeilemaker A, Erpelinck-Verschueren CAJ, Gradowska PL, Meijer R, Cloos J, Biemond BJ, Graux C, van Marwijk Kooy M, Manz MG, Pabst T, Passweg JR, Havelange V, Ossenkoppele GJ, Sanders MA, Schuurhuis GJ, Lowenberg B, Valk PJM (2018) Molecular minimal residual disease in acute myeloid leukemia. N Engl J Med 378(13):1189–1199. https://doi.org/10.1056/NEJMoa1716863
Morita K, Kantarjian HM, Wang F, Yan Y, Bueso-Ramos C, Sasaki K, Issa GC, Wang S, Jorgensen J, Song X, Zhang J, Tippen S, Thornton R, Coyle M, Little L, Gumbs C, Pemmaraju N, Daver N, DiNardo CD, Konopleva M, Andreeff M, Ravandi F, Cortes JE, Kadia T, Jabbour E, Garcia-Manero G, Patel KP, Futreal PA, Takahashi K (2018) Clearance of somatic mutations at remission and the risk of relapse in acute myeloid leukemia. J Clin Oncol 36(18):1788–1797. https://doi.org/10.1200/JCO.2017.77.6757
Zhou YL, Wu LX, Peter Gale R, Wang ZL, Li JL, Jiang H, Jiang Q, Jiang B, Cao SB, Lou F, Sun Y, Wang CC, Liu YR, Wang Y, Chang YJ, Xu LP, Zhang XH, Liu KY, Ruan GR, Huang XJ (2020) Mutation topography and risk stratification for de novo acute myeloid leukaemia with normal cytogenetics and no nucleophosmin 1 (NPM1) mutation or Fms-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD). Br J Haematol 190:274–283. https://doi.org/10.1111/bjh.16526
Levine RL, Valk PJM (2019) Next-generation sequencing in the diagnosis and minimal residual disease assessment of acute myeloid leukemia. Haematologica 104(5):868–871. https://doi.org/10.3324/haematol.2018.205955
Salk JJ, Schmitt MW, Loeb LA (2018) Enhancing the accuracy of next-generation sequencing for detecting rare and subclonal mutations. Nat Rev Genet 19(5):269–285. https://doi.org/10.1038/nrg.2017.117
Hasserjian RP, Steensma DP, Graubert TA, Ebert BL (2020) Clonal hematopoiesis and measurable residual disease assessment in acute myeloid leukemia. Blood 135(20):1729–1738. https://doi.org/10.1182/blood.2019004770
Hirsch P, Tang R, Abermil N, Flandrin P, Moatti H, Favale F, Suner L, Lorre F, Marzac C, Fava F, Mamez AC, Lapusan S, Isnard F, Mohty M, Legrand O, Douay L, Bilhou-Nabera C, Delhommeau F (2017) Precision and prognostic value of clone-specific minimal residual disease in acute myeloid leukemia. Haematologica 102(7):1227–1237. https://doi.org/10.3324/haematol.2016.159681
Debarri H, Lebon D, Roumier C, Cheok M, Marceau-Renaut A, Nibourel O, Geffroy S, Helevaut N, Rousselot P, Gruson B, Gardin C, Chretien ML, Sebda S, Figeac M, Berthon C, Quesnel B, Boissel N, Castaigne S, Dombret H, Renneville A, Preudhomme C (2015) IDH1/2 but not DNMT3A mutations are suitable targets for minimal residual disease monitoring in acute myeloid leukemia patients: a study by the Acute Leukemia French Association. Oncotarget 6(39):42345–42353. https://doi.org/10.18632/oncotarget.5645
Ok CY, Loghavi S, Sui D, Wei P, Kanagal-Shamanna R, Yin CC, Zuo Z, Routbort MJ, Tang G, Tang Z, Jorgensen JL, Luthra R, Ravandi F, Kantarjian HM, DiNardo CD, Medeiros LJ, Wang SA, Patel KP (2019) Persistent IDH1/2 mutations in remission can predict relapse in patients with acute myeloid leukemia. Haematologica 104(2):305–311. https://doi.org/10.3324/haematol.2018.191148
Ferret Y, Boissel N, Helevaut N, Madic J, Nibourel O, Marceau-Renaut A, Bucci M, Geffroy S, Celli-Lebras K, Castaigne S, Thomas X, Terre C, Dombret H, Preudhomme C, Renneville A (2018) Clinical relevance of IDH1/2 mutant allele burden during follow-up in acute myeloid leukemia. A study by the French ALFA group. Haematologica 103(5):822–829. https://doi.org/10.3324/haematol.2017.183525
Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, Kennedy JA, Schimmer AD, Schuh AC, Yee KW, McLeod JL, Doedens M, Medeiros JJF, Marke R, Kim HJ, Lee K, McPherson JD, Hudson TJ, Pan-Leukemia Gene Panel Consortium TH, Brown AMK, Yousif F, Trinh QM, Stein LD, Minden MD, Wang JCY, Dick JE (2014) Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature 506(7488):328–333. https://doi.org/10.1038/nature13038
Press RD, Eickelberg G, Froman A, Yang F, Stentz A, Flatley EM, Fan G, Lim JY, Meyers G, Maziarz RT, Cook RJ (2019) Next-generation sequencing-defined minimal residual disease before stem cell transplantation predicts acute myeloid leukemia relapse. Am J Hematol 94(8):902–912. https://doi.org/10.1002/ajh.25514
Kohlmann A, Nadarajah N, Alpermann T, Grossmann V, Schindela S, Dicker F, Roller A, Kern W, Haferlach C, Schnittger S, Haferlach T (2014) Monitoring of residual disease by next-generation deep-sequencing of RUNX1 mutations can identify acute myeloid leukemia patients with resistant disease. Leukemia 28(1):129–137. https://doi.org/10.1038/leu.2013.239
Onecha E, Linares M, Rapado I, Ruiz-Heredia Y, Martinez-Sanchez P, Cedena T, Pratcorona M, Oteyza JP, Herrera P, Barragan E, Montesinos P, Vela JAG, Magro E, Anguita E, Figuera A, Riaza R, Martinez-Barranco P, Sanchez-Vega B, Nomdedeu J, Gallardo M, Martinez-Lopez J, Ayala R (2019) A novel deep targeted sequencing method for minimal residual disease monitoring in acute myeloid leukemia. Haematologica 104(2):288–296. https://doi.org/10.3324/haematol.2018.194712
Mencia-Trinchant N, Hu Y, Alas MA, Ali F, Wouters BJ, Lee S, Ritchie EK, Desai P, Guzman ML, Roboz GJ, Hassane DC (2017) Minimal Residual Disease Monitoring of Acute Myeloid Leukemia by Massively Multiplex Digital PCR in Patients with NPM1 Mutations. J Mol Diagn 19(4):537–548. https://doi.org/10.1016/j.jmoldx.2017.03.005
Cocciardi S, Dolnik A, Kapp-Schwoerer S, Rucker FG, Lux S, Blatte TJ, Skambraks S, Kronke J, Heidel FH, Schnoder TM, Corbacioglu A, Gaidzik VI, Paschka P, Teleanu V, Gohring G, Thol F, Heuser M, Ganser A, Weber D, Strang E, Kestler HA, Dohner H, Bullinger L, Dohner K (2019) Clonal evolution patterns in acute myeloid leukemia with NPM1 mutation. Nat Commun 10(1):2031. https://doi.org/10.1038/s41467-019-09745-2
Baer MR, Stewart CC, Dodge RK, Leget G, Sule N, Mrozek K, Schiffer CA, Powell BL, Kolitz JE, Moore JO, Stone RM, Davey FR, Carroll AJ, Larson RA, Bloomfield CD (2001) High frequency of immunophenotype changes in acute myeloid leukemia at relapse: implications for residual disease detection (Cancer and Leukemia Group B Study 8361). Blood 97(11):3574–3580. https://doi.org/10.1182/blood.v97.11.3574
Kim B, Kim E, Lee S-T, Cheong J-W, Lyu CJ, Min YH, Choi JR (2020) Detection of recurrent, rare, and novel gene fusions in patients with acute leukemia using next-generation sequencing approaches. Hematol Oncol 38(1):82–88. https://doi.org/10.1002/hon.2709
Mack EKM, Marquardt A, Langer D, Ross P, Ultsch A, Kiehl MG, Mack HID, Haferlach T, Neubauer A, Brendel C (2019) Comprehensive genetic diagnosis of acute myeloid leukemia by next-generation sequencing. Haematologica 104(2):277–287. https://doi.org/10.3324/haematol.2018.194258
Yoest JM, Shirai CL, Duncavage EJ (2020) Sequencing-Based Measurable Residual Disease Testing in Acute Myeloid Leukemia. Front Cell Dev Biol 8(249). https://doi.org/10.3389/fcell.2020.00249
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Carlsen, E.D., Aggarwal, N. & Bailey, N.G. Molecular methods for measurable residual disease in acute myeloid leukemia: where are we and where are we going?. J Hematopathol 14, 3–14 (2021). https://doi.org/10.1007/s12308-020-00440-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-020-00440-6