Abstract
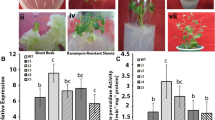
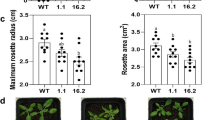
A stress inducible cytosolic ascorbate peroxidase gene (AhcAPX) was ectopically expressed in banana (cv. Grand naine) plants to strengthen their antioxidant capacity. High level of AhcAPX gene transcripts and enzyme suggested constitutive and functional expression of candidate gene in transgenic (TR) plants. The tolerance level of in vitro and in vivo grown TR banana plantlets were assessed against salt and drought stress. The TR banana plants conferred tolerance against the abiotic stresses by maintaining a high redox state of ascorbate and glutathione, which correlated with lower accumulation of H2O2, O ⋅−2 and higher level of antioxidant enzyme (SOD, APX, CAT, GR, DHAR and MDHAR) activities. The efficacy of AhcAPX over-expression was also investigated in terms of different physiochemical attributes of TR and untransformed control plants, such as, proline content, membrane stability, electrolyte leakage and chlorophyll retention. The TR plants showed higher photochemical efficiency of PSII (Fv/Fm), and stomatal attributes under photosynthesis generated reactive oxygen species (ROS) stress. The outcome of present investigation suggest that ectopic expression of AhcAPX gene in banana enhances the tolerance to drought and salt stress by annulling the damage caused by ROS.







Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Badawi GH, Kawano N, Yamauchi Y, Shimada E, Sasaki R, Kubo A, Tanaka K (2004) Over-expression of ascorbate peroxidase in tobacco chloroplasts enhances the tolerance to salt stress and water deficit. Physiol Plant 121(2):231–238
Bartwal A, Arora S (2016) Drought stress-induced enzyme activity and mdar and apx gene expression in tolerant and susceptible genotypes of Eleusine coracana (L.). In Vitro Cell Dev Biol Plant 53(1):41–49
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39(1):205–207
Bernal MP, Remedios DA, Perez OV, Rigo MD, Ramos RA (2016) Assessment three constitutive promoters for GUS expression in rice (Oryza sativa L., var. J-104). Rev Colomb Biotecnol [online] 18(1):81–98
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161(2):559–566
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Cao S, Du XH, Li LH, Liu YD, Zhang L et al (2017) Overexpression of Populus tomentosa cytosolic ascorbate peroxidase enhances abiotic stress tolerance in tobacco plants. Russ J Plant Physiol 64(2):224–234
Chawla S, Jain S, Jain V (2013) Salinity induced oxidative stress and antioxidant system in salt-tolerant and salt-sensitive cultivars of rice (Oryza sativa L.). J Plant Biochem Biotechnol 22(1):27–34
Chen Z, Gallie DR (2004) The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell 16:1143–1162
Chen Y, Cai J, Yang FX, Zhou B, Zhou LR (2015) Ascorbate peroxidase from Jatropha curcas enhances salt tolerance in transgenic Arabidopsis. Genet Mol Res 14(2):4879–4889
Chrysargyris A, Michailidi E, Tzortzakis N (2018) Physiological and biochemical responses of Lavandula angustifolia to salinity under mineral foliar application. Front Plant Sci 9:489
Cicek N, Cakirlar H (2002) The effect of salinity on some physiological and photosynthetic parameters of three different temperatures in six soya bean (Glycine max L. Merr.) cultivars. J Agric Crop Sci 194(1):34–46
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci 2:53
Dbira S, Hassan MA, Gramazio P, Ferchichi A, Prohens VJ, Boscaiu M (2018) Variable levels of tolerance to water stress (drought) and associated biochemical markers in Tunisian barley landraces. Molecules 23(3):613
FAO Climate change and food security: risks and responses (2016) FAO. www.fao.org/publications. ISBN 978-92-5-108998-9
FAOSTAT (2014) Statistics Division, Food and Agriculture Organization of the United Nations. Viale delle Terme di Caracalla. Rome, Italy. www.faostat.fao.org/default.aspx
Foyer CH, Noctor G (2003) Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 119(3):355–364
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155(1):2–18
Fracheboud Y, Haldimann P, Leipner J, Stamp P (1999) Chlorophyll fluorescence as a selection tool for cold tolerance of photosynthesis in maize (Zea mays L.). J Exp Bot 50(338):1533–1540
Gillespie KM, Ainsworth EA (2007) Measurement of reduced, oxidized and total ascorbate content in plants. Nat Protoc 2(4):871–874
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141(2):312–322
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments: a review. Plant Signal Behav 7(11):1456–1466
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125(1):189–198
Hiner AN, Rodríguez-López JN, Arnao MB, Lloyd Raven E, García-Cánovas F, Acosta M (2000) Kinetic study of the inactivation of ascorbate peroxidase by hydrogen peroxide. Biochem J 348(2):321–328
Hossain MA, Nakano Y, Asada K (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25(3):385–395
IPCC (2014a) Climate change 2014: synthesis report. In: Core Writing Team, Pachauri RK, Meyer LA (eds) Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. IPCC, Geneva
IPCC (2014b) Climate change 2014: impacts, adaptation, and vulnerability. Part a: global and sectoral aspects. In: Field CB, Barros VR, Dokken DJ Mach KJ, Mastrandrea MD, Bilir TE, Chatterjee M et al (eds) Contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge and New York
Jaspers P, Kangasjärvi J (2010) Reactive oxygen species in abiotic stress signaling. Plant Physiol 138(4):405–413
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5(1987):387–405
Jiang M, Yang W, Xu J, Chen Q (1993) Active oxygen species damage effect of chlorophyll degradation in rice seedling under osmotic stress. Acta Bot Sin 36(4):289–295
Jozefczak M, Bohler S, Schat HT et al (2015) Both the concentration and redox state of glutathione and ascorbate influence the sensitivity of arabidopsis to cadmium. Ann Bot 116(4):601–612
Kalaji HM, Jajoo A, Oukarroum A et al (2016) Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol Plant 38:102
Karpinski S, Escobar C, Karpinska B, Creissen G, Mullineaux PM (1997) Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 9(4):627–640
Kumar D, Yusuf MA, Singh P, Sardar M, Sarin NB (2014) Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio Protoc 4(8):1–4
Kusumi K (2013) Measuring stomatal density in rice. Bio Protoc 3(9):e753
Lu Z, Liu D, Liu S (2007) Two rice cytosolic ascorbate peroxidases differentially improve salt tolerance in transgenic Arabidopsis. Plant Cell Rep 26(10):1909–1917
Mata CG, Lamattina L (2003) Abscisic acid, nitric oxide and stomatal closure-is nitrate reductase one of the missing links. Trends Plant Sci 8(1):20–26
Matzke AJ, Matzke MA (1998) Position effect and epigenetic silencing of plant transgene. Curr Opin Plant Biol 1(2):142–148
Mehler A (1951) Studies on reactions of illuminated chloroplasts: I. Mechanism of the reduction of oxygen and other hill reagents. Arch Biochem Biophys 33(1):65–77
Meyer P, Saedler H (1996) Homology-dependent gene silencing in plants. Annu Rev Plant Physiol Plant Mol Biol 47:23–48
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ 33(4):453–467
Mittler R, Zilinskas BA (1992) Molecular cloning and characterization of a gene encoding pea cytosolic ascorbate peroxidase. J Biol Chem 267(30):21802–21807
Mittler R, Vanderauwera S, Suzuki N et al (2011) ROS signaling: the new wave. Trends Plant Sci 16(6):300–309
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8(19):4321–4325
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880
Nayyar H, Gupta D (2006) Differential sensitivity of C3 and C4 plants to water deficit stress: association with oxidative stress and antioxidants. Environ Exp Bot 58(1–3):106–113
Negi NP, Shrivastava D, Shekhar S, Sharma V, Sarin NB (2016) Simultaneous overexpression of CuZnSOD and cAPX from Arachis hypogaea leads to salinity stress tolerance in tobacco. In Vitro Cell Dev Biol Plant 52(5):484–491
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Noctor G, Mhamdi A, Foyer CH (2014) The roles of reactive oxygen metabolism in drought: not so cut and dried. Plant Physiol 164(4):1636–1648
Padh H (1990) Cellular function of ascorbic acid biochemistry and cell biology. Biochim Biol Cell 68(10):1166–1173
Pandey P, Singh J, Achary V, Mohan M, Reddy MK (2015) Redox homeostasis via gene families of ascorbate-glutathione pathway. Front Environ Sci 3:25
Pintó-Marijuan M, Munné-Bosch S (2014) Photo-oxidative stress markers as a measure of abiotic stress-induced leaf senescence: advantages and limitations. J Exp Bot 65(14):3845–3857
Polowick PL, Quandt J, Mahon JD (2000) The ability of pea transformation technology to transfer genes into peas adapted to western Canadian growing conditions. Plant Sci 153:161–170
Queval G, Noctor G (2007) A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: application to redox profiling during Arabidopsis rosette development. Anal Biochem 363(1):58–69
Ravi I, Mayil Vaganan M (2016) Abiotic stress tolerance in banana. In: Rao NKS, Shivashankara KS, Laxman RH (eds) Abiotic stress physiology of horticultural crops. Springer, New Delhi, pp 207–222
Rodrigues O, Reshetnyak G, Grondin A et al (2017) Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc Natl Acad Sci USA 114(34):9200–9205
Rustagi A, Jain S, Kumar D, Shekhar S, Jain M, Bhat V, Sarin NB (2015) High efficiency transformation of banana [Musa acuminata L. cv. Matti (AA)] for enhanced tolerance to salt and drought stress through overexpression of a peanut salinity-induced pathogenesis-related class 10 protein. Mol Biotechnol 57(1):27–35
Sairam RK, Srivastava GC (2002) Changes in antioxidant activity in subcellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci 162(2002):897–904
Sambrook J, Fritschi EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 217037:26
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K (2002) Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 53(372):1305–1319
Shrivastava DC, Kisku AV, Saxena M, Deswal R, Sarin NB (2015) Stress inducible cytosolic ascorbate peroxidase (AhcAPX) from Arachis hypogaea cell lines confers salinity and drought stress tolerance in transgenic tobacco. Nucleus 58(1):3–13
Smirnoff N (2000) Ascorbate biosynthesis and function in photoprotection. Philos Trans R Soc B Biol Sci 355(1402):1455–1464
Smith IK, Vierheller TL, Thorne CA (1989) Properties and functions of glutathione reductase in plants. Physiol Plant 77(3):449–456
Soshinkova TN, Radyukina NL, Korolkova DV, Nosov AV (2013) Proline and functioning of the antioxidant system in Thellungiella salsuginea plants and cultured cells subjected to oxidative stress. Russ J Plant Physiol 60(1):41–54
Verslues PE, Sharma S (2010) Proline metabolism and its implications for plant-environment interaction. Arabidopsis Book 8:e0140. https://doi.org/10.1199/tab.0140
Vivancos DP, Faize M, Barba-Espin G et al (2013a) Ectopic expression of cytosolic superoxide dismutase and ascorbate peroxidase leads to salt stress tolerance in transgenic plums. Plant Biotechnol J 11(8):976–985
Vivancos PD, Faize M, Barba-Espin G et al (2013b) Ectopic expression of cytosolic superoxide dismutase and ascorbate peroxidase leads to salt stress tolerance in transgenic plums. Plant Biotechnol J 11(8):976–985
Vivancos DP, Faize L, Nicolás E, Clemente-Moreno MJ et al (2016) Transformation of plum plants with a cytosolic ascorbate peroxidase transgene leads to enhanced water stress tolerance. Ann Bot 117(7):1121–1131
Wairegi LWI, Van Asten PJA, Tenywa MM, Bekunda MA (2010) Abiotic constraints override biotic constraints in highland banana systems. Field Crops Res 117(1):146–153
Wang Y, Wisniewski M, Meilan R, Cui M, Webb R, Fuchigami L (2005) Overexpression of cytosolic ascorbate peroxidase in tomato confers tolerance to chilling and salt stress. J Am Soc Hortic Sci 130(2):167–173
Wang J, Wu B, Yin H et al (2017) Overexpression of CaAPX induces orchestrated reactive oxygen scavenging and enhances cold and heat tolerances in tobacco. Bio Med Res Int 2017:4049534. https://doi.org/10.1155/2017/4049534
Weigel D, Glazebrook J (2006) Transformation of Agrobacterium using the freeze-thaw method. Cold Spring Harb Protoc 2006(7):1031–1036
Xia XJ, Zhou YH, Shi K, Zhou J, Foyer CH, Yu JQ (2015) Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J Exp Bot 66(10):2839–2856
Xu J, Yang J, Duan X, Jiang Y, Zhang P (2014) Increasedexpression of native cytosolic Cu/Zn superoxide dismutase and ascorbateperoxidase improves tolerance to oxidative and chilling stresses in cassava (Manihot esculenta Crantz). BMC Plant Biol 5(14):208
Yadav S, Sharma P, Srivastava A, Desai P, Shrivastava N (2014) Strain specific Agrobacterium-mediated genetic transformation of Bacopa monnieri. J Genet Eng Biotechnol 12(2):89–94
Yamauchi N, Funamoto Y, Shigyo M (2004) Peroxidase mediated chlorophyll degradation in horticultural crops. Phtochem Rev 3(1–2):221–228
Zhang Y (2013) Biological role of ascorbate in plants ascorbic acid in plants. Springer Briefs Plant Sci. https://doi.org/10.1007/978-1-4614-4127-4_2
Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126(4):1438–1448
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shekhar, S., Rustagi, A., Kumar, D. et al. Groundnut AhcAPX conferred abiotic stress tolerance in transgenic banana through modulation of the ascorbate–glutathione pathway. Physiol Mol Biol Plants 25, 1349–1366 (2019). https://doi.org/10.1007/s12298-019-00704-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-019-00704-1