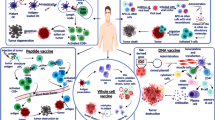
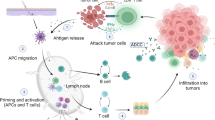
Abstract
Over the past few decades, there has been significant advancement in the field of tumor immunotherapy. For many years vaccination against infectious diseases have been available. On the other hand very few cancer vaccines have been approved for human use. Ideal Cancer vaccines are biological response modifier work by stimulating both humoral and cellular immunity while overcoming the immunological suppression found in tumor. Two types of cancer vaccine: Prophylactic and therapeutic cancer vaccines are recommended for clinical use of individuals. HPV and HBV vaccines are the two widely used preventive vaccine used for treatment of cervical and hepatocellular carcinoma respectively and are approved by Food and Drug Administration (FDA). In therapeutic vaccine only three are approved: Sipuleucel T-cell vaccine for treatment refractory prostatic cancer, BCG vaccine for early bladder cancer and T-VEC for inoperable melanoma. Active ingredient in all cancer vaccines is an antigen. Antigens used for formulating cancer vaccines along with adjuvants optimizes immunogenicity in it. Heterogeneity within and between cancer types, screening and identifying suitable antigen specific to tumors and selection of vaccine delivery platforms are challenges in the development of vaccines. Adoptive cell therapy, Chimeric antigen receptor T cell therapy are recent breakthrough for cancer treatment.

Similar content being viewed by others
References
Ginglen JG, Doyle MQ. Immunization. In: Stat Pearls. Treasure Island (FL): StatPearls Publishing; 2023.
Hajdu SI. A note from history: Landmarks in history of cancer, part 1. Cancer. 2011;117(5):1097–102.
Dobosz P, Dzieciątkowski T. The Intriguing History of Cancer Immunotherapy. Front Immunol. 2019;10:2965.
Oiseth SJ, Aziz MS. Cancer immunotherapy: a brief review of the history, possibilities, and challenges ahead. J Cancer Metastasis Treat. 2017;3:250–61.
Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases 1893. Clin Orthop Relat Res. 1991;262:3–11.
Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180–3.
Decker WK, da Silva RF, Sanabria MH, Angelo LS, Guimarães F, Burt BM, et al. Cancer immunotherapy: historical perspective of a clinical revolution and emerging preclinical animal models. Front Immunol. 2017;8:829.
Beasley RP. Hepatitis B Virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61(10):1942–56.
Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308.
Vesely MD, Schreiber RD. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Ann N Y Acad Sci. 2013;1284(1):1–5.
Liu J, Fu M, Wang M, Wan D, Wei Y, Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J Hematol Oncol. 2022;15(1):28.
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–22.
Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. 2019;38:255.
Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125(26):4017–23.
Tormoen GW, Crittenden MR, Gough MJ. Role of the immunosuppressive microenvironment in immunotherapy. Adv Radiat Oncol. 2018;3:520–6.
Kim SK, Cho SW. The evasion mechanisms of cancer immunity and drug intervention in the tumor microenvironment. Front Pharmacol. 2022;13:868695.
Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17(9):559–72.
Thomas DA, Massagué J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8(5):369–80.
Halle S, Halle O, Förster R. Mechanisms and dynamics of T cell-mediated cytotoxicity in vivo. Trends Immunol. 2017;38(6):432–43.
Zhang Y, Guan XY, Jiang P. Cytokine and chemokine signals of T-cell exclusion in tumors. Front Immunol. 2020;11:594609.
Paston SJ, Brentville VA, Symonds P, Durrant LG. Cancer vaccines, adjuvants, and delivery systems. Front Immunol. 2021;12:627932.
Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14(2):135–46.
Buonaguro L, Petrizzo A, Tornesello ML, Buonaguro FM. Translating tumor antigens into cancer vaccines. Clin Vaccine Immunol CVI. 2011;18(1):23.
Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21(6):360–78.
Weon JL, Potts PR. The MAGE protein family and cancer. Curr Opin Cell Biol. 2015;37:1–8.
Parkhurst MR, Fitzgerald EB, Southwood S, Sette A, Rosenberg SA, Kawakami Y. Identification of a shared HLA-A*0201-restricted T-cell epitope from the melanoma antigen tyrosinase-related protein 2 (TRP2). Cancer Res. 1998;58:4895–901.
Correale P, Walmsley K, Nieroda C, Zaremba S, Zhu M, Schlom J, et al. In vitro generation of human cytotoxic T lymphocytes specific for peptides derived from prostate-specific antigen. J Natl Cancer Inst. 1997;89:293–300.
Lam KW, Li CY, Yam LT, Sun T, Lee G, Ziesmer S. Improved immunohistochemical detection of prostatic acid phosphatase by a monoclonal antibody. Prostate. 1989;15:13–21.
Disis ML, Wallace DR, Gooley TA, Dang Y, Slota M, Lu H, et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol. 2009;27:4685–92.
Srivastava PK. Neoepitopes of cancers: looking back, looking ahead. Cancer Immunol Res. 2015;3(9):969–77.
Peng M, Mo Y, Wang Y, Wu P, Zhang Y, Xiong F, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18(1):128.
Tsao SW, Tramoutanis G, Dawson CW, Lo AKF, Huang DP. The significance of LMP1 expression in nasopharyngeal carcinoma. Cancer Biol. 2002;12:473–87.
Yarchoan M, Gane E, Marron TU, Rochestie S, Cooch N, Peters J, et al. Personalized DNA neoantigen vaccine in combination with plasmid IL-12 and pembrolizumab for the treatment of patients with advanced hepatocellular carcinoma. J Clin Oncol. 2021;39(15):2680.
Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur V, Stevanovic S, Gouttefangeas C, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–5.
Flores JE, Thompson AJ, Ryan M, Howell J. The global impact of hepatitis B Vaccination on hepatocellular carcinoma. Vaccines (Basel). 2022;10(5):793.
Chang MH. Hepatitis B virus and cancer prevention. Recent Results Cancer Res. 2011;188:75–84.
Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Sm Lin, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101(19):1348–55.
Li Y, Xu C. Human papillomavirus-related cancers. Adv Exp Med Biol. 2017;1018:23–34.
Kaarthigeyan K. Cervical cancer in India and HPV vaccination. Indian J Med Paediatr Oncol. 2012;33(1):7–12.
World Health Organization. Human papillomavirus vaccines: WHO position paper, May 2017–Recommendations. Vaccine. 2017;35(43):5753–5.
History of vaccines. Cancer vaccines and Immunotherapy (2018) Available online at : https://www.historyofvaccines.org/content/articles/cancervaccinesandimmunotherapy (Accessed 19 Nov 2023).
de Gruijl TD, van den Eertwegh AJ, Pinedo HM, Scheper RJ. Whole-cell cancer vaccination: from autologous to allogeneic tumor- and dendritic cell-based vaccines. Cancer Immunol Immunother. 2008;57:1569–77.
Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24(19):3089–94.
Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52.
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22.
Anassi E, Ndefo UA. Sipuleucel-T (Provenge) injection: the first immunotherapy agent (vaccine) for hormone-refractory prostate cancer. Pharm Ther. 2011;36(4):197.
Luca S, Mihaescu T. History of BCG vaccine. Maedica (Bucur). 2013;8(1):53.
Lamm DL, Thor DE, Harris SC, Reyna JA, Stogdill VD, Radwin HM. Bacillus Calmette-Guerin immunotherapy of superficial bladder cancer. J Urol. 1980;124(1):38–42.
Cardillo F, Bonfim M, da Silva Vasconcelos Sousa P, Mengel J, Ribeiro Castello-Branco LR, Pinho RT. Bacillus Calmette-guérin immunotherapy for cancer. Vaccines (Basel). 2021;9(5):439.
Kaufman HL, Shalhout SZ, Iodice G. Talimogene laherparepvec: moving from first-in-class to best-in-class. Front Mol Biosci. 2022;9:834841.
Ferrucci PF, Pala L, Conforti F, Cocorocchio E. Talimogene laherparepvec (T-VEC): an intralesional cancer immunotherapy for advanced melanoma. Cancers (Basel). 2021;13(6):1383.
Fu C, Zhou L, Mi QS, Jiang A. DC-based vaccines for cancer immunotherapy. Vaccines (Basel). 2020;8(4):706.
Srinivasan P, Wu X, Basu M, Rossi C, Sandler AD. PD-L1 checkpoint inhibition and anti-CTLA-4 whole tumor cell vaccination counter adaptive immune resistance: a mouse neuroblastoma model that mimics human disease. PLoS Med. 2018;15(1):e1002497.
Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, van der Burg SH, Offringa R. Superior induction of anti-tumor CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur J Immunol. 2008;38(4):1033–42.
Southwood S, Sidney J, Kondo A, del Guercio MF, Appella E, Hoffman S, et al. Several common HLA-DR types share largely overlapping peptide binding repertoires. J Immunol. 1998;160(7):3363–73.
Tashiro H, Brenner MK. Immunotherapy against cancer-related viruses. Cell Res. 2017;27(1):59–73.
Raja J, Ludwig JM, Gettinger SN, Schalper KA, Kim HS. Oncolytic virus immunotherapy: future prospects for oncology. J Immunother Cancer. 2018;6:1–13.
Russell SJ, Barber GN. Oncolytic viruses as antigen-agnostic cancer vaccines. Cancer Cell. 2018;33(4):599–605.
Pham T, Roth S, Kong J, Guerra G, Narasimhan V, Pereira L, et al. An Update on immunotherapy for solid tumors: a review. Ann Surg Oncol. 2018;25:3404–12.
Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev. 2011;239:62–84.
MacGregor RR, Boyer JD, Ugen KE, Lacy KE, Gluckman SJ, Bagarazzi ML, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis. 1998;178(1):92–100.
Tiptiri-Kourpeti A, Spyridopoulou K, Pappa A, Chlichlia K. DNA vaccines to attack cancer: strategies for improving immunogenicity and efficacy. Pharmacol Ther. 2016;165:32–49.
Bloom K, van den Berg F, Arbuthnot P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2021;28:117–29.
Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–79.
Sahin U, Kariko K, Tureci O. mRNA-based therapeutics—developing a new class of drugs. Nat Rev Drug Discov. 2014;13(10):759–80.
Donninger H, Li C, Eaton JW, Yaddanapudi K. Cancer vaccines: promising therapeutics or an unattainable dream. Vaccines (Basel). 2021;9(6):668.
Bayan CY, Lopez AT, Gartrell RD, Komatsubara KM, Bogardus M, Rao N, et al. The role of oncolytic viruses in the treatment of melanoma. Curr Oncol Rep. 2018;20(10):80.
Song H, Zhong LP, He J, Huang Y, Zhao YX. Application of Newcastle disease virus in the treatment of colorectal cancer. World J Clin Cases. 2019;7(16):2143–54.
Berd D, Maguire HC Jr, McCue P, Mastrangelo MJ. Treatment of metastatic melanoma with an autologous tumor-cell vaccine: clinical and immunologic results in 64 patients. J Clin Oncol. 1990;8(11):1858–67.
Möller P, Sun Y, Dorbic T, Alijagic S, Makki A, Jurgovsky K, et al. Vaccination with IL-7 gene-modified autologous melanoma cells can enhance the anti-melanoma lytic activity in peripheral blood of patients with a good clinical performance status: a clinical phase I study. Br J Cancer. 1998;77(11):1907–16.
Sosman JA, Sondak VK. Melacine: an allogeneic melanoma tumor cell lysate vaccine. Expert Rev Vaccines. 2003;2(3):353–68.
Amato RJ. Vaccine therapy for renal cell carcinoma. Rev Urol. 2003;5(2):65–71.
Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA. 1989;86:10024–8.
NIH CAR T-Cell Therapy Approved for Some Children and Young Adults with Leukemia (2017) Available online at: https://www.cancer.gov/news-events/cancer-currents-blog/2017/tisagenlecleucel-fda-childhood-leukemia Accessed 19 Nov 2023
Sun Z, Zhao H, Ma L, Shi Y, Ji M, Sun X, et al. The quest for nanoparticle-powered vaccines in cancer immunotherapy. J Nanobiotechnol. 2024;22(1):61.
Yao M, Liu X, Qian Z, Fan D, Sun X, Zhong L, et al. Research progress of nanovaccine in anti-tumor immunotherapy. Front Oncol. 2023;13:1211262.
Baker DJ, Arany Z, Baur JA, Epstein JA, June CH. CAR T therapy beyond cancer: the evolution of a living drug. Nature. 2023;619:707–15.
Saleh R, Elkord E. Acquired resistance to cancer immunotherapy: role of tumor-mediated immunosuppression. Semin Cancer Biol. 2020;65:13–27.
Yang L, Li A, Lei Q, Zhang Y. Tumor-intrinsic signaling pathways: key roles in the regulation of the immunosuppressive tumor microenvironment. J Hematol Oncol. 2019;12(1):125.
Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression—implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16(6):356–71.
Mazzarella L, Duso BA, Trapani D, Belli C, D’Amico P, Ferraro E, et al. The evolving landscape of ‘next-generation’ immune checkpoint inhibitors: a review. Eur J Cancer. 2019;117:14–31.
Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGF beta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554(7693):544–8.
Funding
Nil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sarangi, R., Mishra, S. & Mahapatra, S. Cancer Vaccines: A Novel Revolutionized Approach to Cancer Therapy. Ind J Clin Biochem (2024). https://doi.org/10.1007/s12291-024-01201-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12291-024-01201-3