Abstract
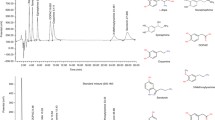
Biogenic amine neurotransmitters such as serotonin and dopamine are essential for signaling in both central and peripheral nervous system. Their metabolism is a multistep pathway and any defect in this results in alteration in metabolites of serotonin 5-Hydroxyindole acetic acid (5HIAA) and dopamine homovanillic acid (HVA) and 3-O-Methyl Dopa (3-OMD). Estimation of these metabolites in cerebrospinal fluid (CSF) assists in diagnosis of neurotransmitter defects. Their estimation is technically demanding and is currently available only in referral centers. We aimed to optimize a method for analysis of 5HIAA, HVA and 3-OMD. A high performance liquid chromatography (HPLC) method with electro chemical detector (ECD) was standardized for estimation. Analysis for method validation, reference range verification and clinical correlation was performed. Linearity obtained for 5-HIAA, HVA and 3-OMD was 65.35–2615.0 nmoles/l, 68.62–2745.0 nmoles/l and 236.5–4730.0 nmoles/l respectively. The coefficient of variation for internal quality controls ranged from 5 to 14% and the external proficiency testing samples (n = 16) were within peer group range. CSF metabolite levels of samples for reference range analysis overlapped with age matched ranges reported in literature. Among the 40 suspected patients analyzed for clinical testing four were found to have a neurotransmitter defect. These patients were then confirmed with molecular testing and clinical correlation. The method is validated and can be adapted in a clinical laboratory with analytical competence in HPLC.




Similar content being viewed by others
References
Neurotransmitters. Encyclopaedia Britannica. Encyclopaedia Britannica online. Encyclopaedia Britannica Inc.,2018.Web.04 Jul. 2018. <https://www.britannica.com/science/neurotransmitter>
Ng J, Papandreou A, Heales S, Kurian M. Monoamine neurotransmitter disorders—clinical advances and future perspectives. Nat Rev Neurol. 2015;11(10):567–84.
Rodan L, Gibson K, Pearl P. Clinical use of CSF neurotransmitters. PediatrNeurol. 2015;53(4):277–86.
Doummar D, Moussa F, Nougues M, Ravelli C, Louha M, Whalen S, et al. Monoamine neurotransmitters and movement disorders in children and adults. Revue Neurologique. 2018;174(9):581–8.
Ormazabal A, García-Cazorla A, Fernández Y, Fernández-Álvarez E, Campistol J, Artuch R. HPLC with electrochemical and fluorescence detection procedures for the diagnosis of inborn errors of biogenic amines and pterins. J Neurosci Methods. 2005;142(1):153–8.
Jung-Klawitter S, Kuseyri HO. Analysis of catecholamines and pterins in inborn errors of monoamine neurotransmitter metabolism—from past to future. Cells. 2019;8(8):867.
Burlina A, Celato A. The utility of CSF for the diagnosis of primary and secondary monoamine neurotransmitter deficiencies. J Int Federation ClinChem Lab Med. 2017;28:64–76.
World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4.
Hyland K. Clinical utility of monoamine neurotransmitter metabolite analysis in cerebrospinal fluid. ClinChem. 2008;54(4):633–41.
Honeychurch K. Review: the application of liquid chromatography electrochemical detection for the determination of drugs of abuse. Separations. 2016;3(4):28.
ICH Topic Q 2 (R1) Validation of analytical procedures: text and methodology. CPMP/ICH/381/95,1995
Guideline on bioanalytical method validation. European Medicines agency, EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2**,2011
Ravichandran V, Shalini S. Validation of analytical methods—strategies & importance. Int J Pharmacy Pharm Sci. 2010;2(3):18–22.
Blau N. Physician's guide to the laboratory diagnosis of metabolic diseases. First edition.London: Chapman and Hall medical; 1996: p 79–99.
Kuster A, Arnoux J, Barth M, et al. Diagnostic approach to neurotransmitter monoamine disorders: experience from clinical, biochemicals and genetic profiles. J Inherit Meta Dis. 2018;41(1):129–39.
Opladen T, Cortès-Saladelafont E, Mastrangelo M, Horvath G, Pons R, Lopez-Laso E, et al. The international working group on neurotransmitter related disorders (iNTD): a worldwide research project focused on primary and secondary neurotransmitter disorders. Mol Gene Metabol Reports. 2016;9:61–6.
Mercimek-Mahmutoglu S, Sidky S, Hyland K, Patel J, Donner E, Logan W, et al. Prevalence of inherited neurotransmitter disorders in patients with movement disorders and epilepsy: a retrospective cohort study. Orphanet J Rare Dis. 2015;10(1):12.
Batllori M, Molero-Luis M, Ormazabal A, Casado M, Sierra C, García-Cazorla A, et al. Analysis of human cerebrospinal fluid monoamines and their cofactors by HPLC. Nat Protoc. 2017;12(11):2359–75.
Acknowledgements
Resources: We acknowledge support extended by the Development committee, National Health and Education Society for funding the study, clinicians for referring patients and patients and their families for providing study samples.
Proficiency Testing: P. D. Hinduja Hospital and MRC acknowledge the use of data derived from ERNDIM EQA materials in this paper. The use of ERNDIM EQA materials does not imply that ERNDIM endorses the methods used or the scientific validity of the findings in this paper. ERNDIM (www.erndim.org) is an independent, not for profit foundation that provides EQA schemes in the field of inborn errors of metabolism with the aim of improving diagnosis, treatment and monitoring of inherited metabolic diseases.
Funding
Development committee, National Health and Education Society.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lokhande, R.V., Bhagure, G.R., Dherai, A.J. et al. Analytical Method Validation for Estimation of Neurotransmitters (Biogenic Monoamines) from Cerebrospinal Fluid Using High Performance Liquid Chromatography. Ind J Clin Biochem 37, 85–92 (2022). https://doi.org/10.1007/s12291-020-00949-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-020-00949-8