Abstract
Background
Transfusion Transmitted infections(TTI) are of significant concern for blood safety. The thalassemia patients who receive multiple transfusions are at an increased risk of TTIs and the Nucleic Acid Test (NAT ) has been advocated for safe blood. Though NAT can reduce the window period compared to serology, cost is a constraint.
Methods
The thalassemia patient and NAT yield data from the centralized NAT lab in AIIMS Jodhpur was evaluated for cost-effectiveness using the Markov model. The incremental cost-effectiveness ratio (ICER) was calculated by dividing the difference between the cost for NAT and the cost of medical management of TTI-related complications by the product of the difference in utility value of a TTI health state with time and Gross National Income(GNI) per capita.
Results
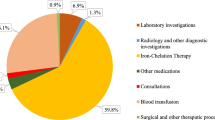
Out of the 48,762 samples tested by NAT, 43 samples were discriminated NAT yield all of which were reactive for Hepatitis B (NAT yield of 1:1134). There was no HCV and HIV NAT yield despite HCV being the most prevalent TTI in this population. The cost of this intervention was INR 5,85,14,400. The number of lifetime QALY saved was 1.38 years. The cost of medical management is INR 82,19,114. Therefore the ICER for intervention is INR 3,64,45,860 per QALY saved which is 274 times the GNI per capita of India.
Conclusions
The provision of IDNAT-tested blood for thalassemia patients in Rajasthan state was not found to be cost-effective. Measures to bring down the cost or alternative options to increase blood safety should be explored.

Similar content being viewed by others
References
Sharma P, Jora R, Garg A, TO STUDY THE PREVALENCE OF TRANSFUSION, TRANSMITTED INFECTIONS AMONGST MULTI-TRANSFUSED THALASSEMIC PATIENTS OF WESTERN RAJASTHAN (2016) IJARR 1(10):44–51
Gorakshakar AC, Ghosh K (2013) Transfusion transmitted infections in Indian thalassemics: a perspective. Indian J Hematol Blood Transfus 29(3):189–190. doi:https://doi.org/10.1007/s12288-012-0168-5/
2016DrugsandCosmeticsAct1940Rules1945.pdf. [cited 2021 Jun 18]. Available from: https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/Pdf-documents/acts_rules/2016DrugsandCosmeticsAct1940Rules1945.pdf
Makroo RN, Chowdhry M, Bhatia A, Arora B, Rosamma NL. Hepatitis B core antibody testing in Indian blood donors: A double-edged sword!Asian J Transfus Sci. 2012Jan; 6(1):10–3. doi: https://doi.org/10.4103/0973-6247.95043
Gupta E, Bajpai M, Choudhary A, Hepatitis C (2014) virus: Screening, diagnosis, and interpretation of laboratory assays. Asian J Transfus Sci. ;8(1):19–25. doi:https://doi.org/10.4103/0973-6247.126683
Kumar Barik S, Mohanty KK, Bisht D, Joshi B, Jena S, Tripathy SP(2018) An Overview of Enzyme Immunoassay: The Test Generation Assay in HIV/AIDS Testing.J AIDS Clin Res; 09(03)
Hans R, Marwaha N (2014) Nucleic acid testing-benefits and constraints. Asian J Transfus Sci 8(1):2–3. doi:https://doi.org/10.4103/0973-6247.126679
Ghosh K, Mishra K (2017) Nucleic acid amplification testing in Indian blood banks: A review with perspectives. Indian J Pathol Microbiol 60:313–318
Kumar R, Gupta S, Kaur A, Gupta M (2015) Individual donor-nucleic acid testing for human immunodeficiency virus-1, hepatitis C virus and hepatitis B virus and its role in blood safety. Asian J Transfus Sci 9(2):199–202
Datta S, Khillan K, Ranjan V, Wattal C (2019) Nucleic acid amplification test: Bridging the gap in blood safety & re-evaluation of blood screening for cryptic transfusion-transmitted infection among Indian donors. Indian J Med Res 149(3):389
Sinha D, Explained (2021) : Despite Govt Claims, India’s Health Budget Only Around 0.34% of GDP. The Wire Science. Available from: https://science.thewire.in/health/union-health-budget-nirmala-sitharaman-covid-19-pmasby-allocation-gdp-expert-analysis/ [accessed on 2021 Jun 6]
Out-of-pocket expenditure (% of current health expenditure) - India | Data. Available from: https://data.worldbank.org/indicator/SH.XPD.OOPC.CH.ZS?end=2018&locations=IN&start=2000&view=chart [accessed on 2021 Jun 6]
Chordiya K, Katewa V, Sharma P, Deopa B, Katewa S (2018 Nov) Quality of Life (QoL) and the Factors Affecting it in Transfusion-dependent Thalassemic Children. Indian J Pediatr 85(11):978–983
Rajasthan witnesses 10% increase of children with Thalassaemia every year : Health minister | Jaipur News - Times of India. Available from: https://timesofindia.indiatimes.com/city/jaipur/rajasthan-witnesses-10-increase-of-children-with-thalassaemia-every-year-health-minister/articleshow/47205092.cms [accessed on 2021 Jun 18]
Experts, patients demand access to standardised blood screening technology. https://www.outlookindia.com/. Available from: https://www.outlookindia.com/newsscroll/experts-patients-demand-access-to-standardised-blood-screening-technology/1682188 [accessed on 2021 Jun 18]
Moirangthem A, Phadke SR (2018 Feb) Socio-demographic Profile and Economic Burden of Treatment of Transfusion Dependent Thalassemia. Indian J Pediatr 85(2):102–107
Dhanya R, Agarwal RK, Sedai A, Kumari A, Parmar L, Hegde S et al. Assessment of Mortality and Its Associated Risk Factors in Patients with Transfusion Dependent Thalassemia in India. Blood. 2019 Nov 13;134(Supplement_1):973–973. https://doi.org/10.1182/blood-2019-125151
Vitrano A, Calvaruso G, Lai E, Colletta G, Quota A, Gerardi C et al (2017) The era of comparable life expectancy between thalassaemia major and intermedia: Is it time to revisit the major-intermedia dichotomy? Br J Haematol 176(1):124–130. https://doi.org/10.1111/bjh.14381
Balasundaram P, Tiwari V, Sherin Raj T(2020) Cost of treatment and consequences for chronic hepatitis B and C virus infection at a tertiary care hospital in Delhi. Indian J Public Health. ;64(4):409. Available from: http://www.ijph.in/text.asp?2020/64/4/409/303097
Fattovich G (2003) Natural History and Prognosis of Hepatitis B. Semin Liver Dis 23(1):047–58. DOI:https://doi.org/10.1055/s-2003-37590
Osborne RH, De Abreu Lourenço R, Dalton A, Houltram J, Dowton D, Joshua DE et al (2007 Nov) Quality of Life Related to Oral versus Subcutaneous Iron Chelation: A Time Trade-off Study. Value in Health 10(6):451–456
Woo G, Tomlinson G, Yim C, Lilly L, Therapondos G, Wong DK et al(2012) Jul;26(7):445–51 Health state utilities and quality of life in patients with hepatitis B. Can J Gastroenterol. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3395446/
Edejer TT-T, Organization WH (eds) (2003) Making choices in health: WHO guide to cost-effectiveness analysis. World Health Organization, Geneva, p 312
Makroo RN, Chowdhry M, Bhatia A, Antony M(2015) Evaluation of the Procleix Ultrio Plus ID NAT assay for detection of HIV 1, HBV and HCV in blood donors. Asian J Transfus Sci. 9(1):29–30. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4339927/
Chigurupati P, Murthy KS(2015) Automated nucleic acid amplification testing in blood banks: An additional layer of blood safety. Asian J Transfus Sci. 9(1):9–11. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4339944/
Arora S, Doda V, Kirtania T(2015) Apr;13(2):227–32 Sensitivity of individual donor nucleic acid testing (NAT) for the detection of hepatitis B infection by studying diluted NAT yield samples. Blood Transfus. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4385070/
Chatterjee K, Coshic P, Borgohain M, Premchand null, Thapliyal RM, Chakroborty S et al (2012 Aug) Individual donor nucleic acid testing for blood safety against HIV-1 and hepatitis B and C viruses in a tertiary care hospital. Natl Med J India 25(4):207–209
Pandey P, Tiwari AK, Dara RC, Rawat GS, Negi A, Raina V(2016) Apr;54(2):242–7 Confirmation and follow up of initial “NAT yields”: Prospective study from a tertiary healthcare center in India. Transfusion and Apheresis Science. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1473050215001470
Agarwal N, Chatterjee K, Coshic P, Borgohain M(2013) Dec;49(3):482–4 Nucleic acid testing for blood banks: An experience from a tertiary care centre in New Delhi, India. Transfusion and Apheresis Science. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1473050213000803
Mittal K, Abrol P, Yadav J. Prevalence of transfusion transmitted infections amongst multiple blood transfused patients of β-thalassemia major in a tertiary care hospital. Int J Res Med Sci. 2016 Dec 19;5(1):181. Available from: http://www.msjonline.org/index.php/ijrms/article/view/364
Sidhu M, Meenia R, Yasmeen I, Sawhney V, Dutt N(2015) Prevalence of transfusion-transmitted infections in multiple blood transfused thalassemia patients: A report from a tertiary care center in North India. Annals of Tropical Medicine and Public Health. Sep 1;8(5):202. Available from: https://www.atmph.org/article.asp?issn=17556783;year=2015;volume=8;issue=5;spage=202;epage=205;aulast=Sidhu;type=0
Shrivastava M, Mishra S (2017) Nucleic Acid Amplification Testing (NAT): An Innovative Diagnostic Approach for Enhancing Blood Safety. Natl J Lab Med 6:6
Seifried E, Schmidt M(2016) Transfusion-transmitted virus infections (TTVIs). In: Rossi’s Principles of Transfusion Medicine. John Wiley & Sons, Ltd; p. 581–98. Available from: https://onlinelibrary.wiley.com/doi/abs/https://doi.org/10.1002/9781119013020.ch51
Seo DH, Whang DH, Song EY, Han KS(2015) Occult hepatitis B virus infection and blood transfusion. World J Hepatol. Mar 27;7(3):600–6. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4381183/
Murhekar MV, Santhosh Kumar M, Kamaraj P, Khan SA, Allam RR, Barde P et al. Hepatitis-B virus infection in India: Findings from a nationally representative serosurvey, 2017-18. International Journal of Infectious Diseases. 2020 Nov 1;100:455–60. Available from: https://www.sciencedirect.com/science/article/pii/S1201971220307128
Su T-H, Chen P-J(2012) Sep;1(9):e27 Emerging hepatitis B virus infection in vaccinated populations: a rising concern? Emerg Microbes Infect. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3630933/
Chandra T, Rizvi SNF, Agarwal D(2014) Decreasing Prevalence of Transfusion Transmitted Infection in Indian Scenario. The Scientific World Journal. ;2014:1–4. Available from: http://www.hindawi.com/journals/tswj/2014/173939/
Makroo RN, Hegde V, Chowdhry M, Bhatia A, Rosamma NL(2015) Sep;142(3):317–22 Seroprevalence of infectious markers & their trends in blood donors in a hospital based blood bank in north india. Indian J Med Res. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4669867/
Mafirakureva N, Mapako T, Khoza S, Emmanuel JC, Marowa L, Mvere D et al(2016) Dec;56(12):3101–11 Cost effectiveness of adding nucleic acid testing to hepatitis B, hepatitis C, and human immunodeficiency virus screening of blood donations in Zimbabwe: ID-NAT FOR SCREENING BLOOD DONATIONS. Transfusion. Available from: https://doi.org/10.1111/trf.13858
Davidson T, Ekermo B, Gaines H, Lesko B, Åkerlind B(2011) Feb;51(2):421–9 The cost-effectiveness of introducing nucleic acid testing to test for hepatitis B, hepatitis C, and human immunodeficiency virus among blood donors in Sweden: COST-EFFECTIVENESS OF NAT. Transfusion. Available from: https://doi.org/10.1111/j.1537-2995.2010.02877.x
Jackson BR, Busch MP, Stramer SL, AuBuchon JP(2003) Jun;43(6):721–9 The cost-effectiveness of NAT for HIV, HCV, and HBV in whole-blood donations. Transfusion. Available from: https://doi.org/10.1046/j.1537-2995.2003.00392.x
Custer B, Janssen MP, Hubben G, Vermeulen M, van Hulst M(2017) Aug;112(6):526–34 Development of a web-based application and multicountry analysis framework for assessing interdicted infections and cost-utility of screening donated blood for HIV, HCV and HBV. Vox Sang. Available from: https://doi.org/10.1111/vox.12538
Van Hulst M, Hubben GAA, Sagoe KWC, Promwong C, Permpikul P, Fongsatitkul L et al. BLOOD DONORS AND BLOOD COLLECTION: Web interface-supported transmission risk assessment and cost-effectiveness analysis of postdonation screening: a global model applied to Ghana, Thailand, and the Netherlands: WEB INTERFACE-SUPPORTED DECISION MAKING. Transfusion. 2009 Aug 25;49(12):2729–42. Available from: https://doi.org/10.1111/j.1537-2995.2009.02351.x
Colah R, Italia K, Gorakshakar A(2017) Burden of thalassemia in India: The road map for control. Pediatric Hematology Oncology Journalƒ. Dec 1;2(4):79–84. Available from: https://www.sciencedirect.com/science/article/pii/S2468124517300748
John MJ, Jyani G, Jindal A, Mashon RS, Mathew A, Kakkar S et al(2018) Oct;24(10):2119–26 Cost Effectiveness of Hematopoietic Stem Cell Transplantation Compared with Transfusion Chelation for Treatment of Thalassemia Major. Biology of Blood and Marrow Transplantation. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1083879118301861
Acknowledgements
We would like to acknowledge the contribution of Mr. Manish Choudhary, State Coordination Officer (Blood Cell), NHM Rajasthan and Mr. Tousif Ashraf, Senior Application specialist, Hemogenomics private limited, India for their contribution in implementation of centralized NAT testing and data collection for the project.
Funding
The project for centralized NAT testing has received funding from National Health Mission, Rajasthan.
Author information
Authors and Affiliations
Contributions
Study conception and design: Dr. Puneeth Babu Anne, Dr. Archana Bajpayee, Dr. Sanjeev Misra, Dr. Mahendra Kumar Garg. Data collection: Dr. Puneeth Babu Anne, Dr. Sunita Bundas, Dr. Manju Bohra. Analysis and interpretation of results: Dr. Puneeth Babu Anne, Dr. Archana Bajpayee, Dr. Suresh Kumar Sharma. Draft manuscript preparation: Dr. Puneeth Babu Anne, Dr. Anubhav Gupta. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Disclaimer
The views expressed in the submitted article are his or her own and not an official position of the institution or funder.
Disclosure of conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anne, P.B., Gupta, A., Misra, S. et al. Economic Evaluation of Nucleic Acid Testing for Screening of Blood Donations for Thalassemia Patients (ECONAT) in Western India. Indian J Hematol Blood Transfus 39, 317–324 (2023). https://doi.org/10.1007/s12288-022-01564-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-022-01564-8