Abstract
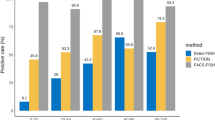
Multiple myeloma (MM) is an uncontrolled proliferation of plasma cells and these cells play an important role in the immune system. In this research, we retrospectively analyzed cytogenetic abnormalities in 381 patients with MM. Conventional cytogenetic analysis was successful in 354 patients (92.9%). Chromosomal abnormalities were detected in 31.9% (113/354) and 45.8% (116/253) of patients screened with conventional cytogenetics and FISH, respectively. Of 113 patients with chromosomal abnormalities, 31 patients (27.4%) had hyperdiploid and 26 of 31 patients with hyperdiploidy had both numerical and structural anomalies. On the other hand, non-hyperdiploidy was observed in 62 patients (54.8%). The most common gains of chromosomes were 15, 9, 19 followed by 3, 5, 11, and 21. Whole chromosome losses were also frequent involving Y, 13 and 22 chromosomes. In our patients, 1q gain was determined in a total of 25 patients (22%), including 7 structural abnormalities and 19 unbalanced translocations causing complete or partial duplication of the long arm of chromosome 1. Although the breakpoints were different, t(1;5) balanced translocation and unbalanced translocations of t(1;2), t(1;3), t(1;7), t(1;16) and t(1;19) were observed twice. The most common structural abnormality was the deletion of the short arm of chromosome 13 (13q) or monosomy of chromosome 13 (-13) (24.1%, 61/253) in patients evaluated by FISH. Deletion involving chromosome 17p (del 17p) or monosomy of chromosome 17 (-17) were found in 31 (12.3%) patients. Translocations involving IgH regions were as follows: t(11;14)(q13;q32.33) in 22 (8.7%), t(4;14)(p16.3;q32.33) in 22 (8.7%) and t(14;16)(q32.33;q23.1) in 2 (0.8%) patients. In addition, t(14;17)(q32;q21) translocation was detected in a multiple myeloma patient for the first time in this study. There are a limited number of large study groups including both cytogenetic and FISH findings in MM patients. As the number of these studies increases, it is thought that new cytogenetic data can be guiding especially in clinical risk determination.



Similar content being viewed by others
References
Palumbo A, Anderson K (2011) Multiple myeloma. N Engl J Med 364(11):1046–1060
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68(1):7–30
Rajkumar SV, Gahrton G, Bergsagel PL (2011) Approach to the treatment of multiple myeloma: a clash of philosophies. Blood 118(12):3205–3211
Fairfield H, Falank C, Avery L, Reagan MR (2016) Multiple myeloma in the marrow: pathogenesis and treatments. Ann N Y Acad Sci 1364(1):32–51
San-Miguel JF, Mateos MV (2011) Can multiple myeloma become a curable disease? Haematologica 96(9):1246–1248
Avet-Loiseau H, Soulier J, Fermand JP, Yakoub-Agha I, Attal M, Hulin C et al (2010) Impact of high-risk cytogenetics and prior therapy on outcomes in patients with advanced relapsed or refractory multiple myeloma treated with lenalidomide plus dexamethasone. Leukemia 24(3):623–628
Laubach JP, Schlossman RL, Mitsiades CS, Anderson KC, Richardson PG (2011) Thalidomide, lenalidomide and bortezomib in the management of newly diagnosed multiple myeloma. Expert Rev Hematol 4(1):51–60
Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK et al (2009) International myeloma working group molecular classification of multiple myeloma: spotlight review. Leukemia 23(12):2210–2221
Kapoor P, Fonseca R, Rajkumar SV, Sinha S, Gertz MA, Stewart AK et al (2010) Evidence for cytogenetic and fluorescence in situ hybridization risk stratification of newly diagnosed multiple myeloma in the era of novel therapies. Mayo Clin Proc 85(6):532–537
Terpos E, Eleutherakis-Papaiakovou V, Dimopoulos MA (2006) Clinical implications of chromosomal abnormalities in multiple myeloma. Leuk Lymphoma 47(5):803–814
Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC et al (2016) Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood 127(24):2955–2962
Nemec P, Zemanova Z, Kuglik P, Michalova K, Tajtlova J, Kaisarova P et al (2012) Complex karyotype and translocation t(4;14) define patients with high-risk newly diagnosed multiple myeloma: results of CMG2002 trial. Leuk Lymphoma 53(5):920–927
Sawyer JR (2011) The prognostic significance of cytogenetics and molecular profiling in multiple myeloma. Cancer Genet 204(1):3–12
Viguié F (2004) Multiple myeloma. Atlas Genet Cytogenet Oncol Haematol 8:255–257
Rajkumar SV, Fonseca R, Dewald GW, Therneau TM, Lacy MQ, Kyle RA et al (1999) Cytogenetic abnormalities correlate with the plasma cell labeling index and extent of bone marrow involvement in myeloma. Cancer Genet Cytogenet 113(1):73–77
International Myeloma Working Group (2003) Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 121(5):749–757
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV et al (2014) International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15(12):e538–e548
Mikhael JR, Dingli D, Roy V, Reeder CB, Buadi FK, Hayman SR et al (2013) Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc 88(4):360–376
McGowan-Jordan J, Simons A, Schmid M (2016) ISCN 2016: an international system for human cytogenomic nomenclature (2016) cytogenetic and genome research, vol 149, no 1–2, 1st edn
Munshi NC, Anderson KC, Bergsagel PL, Shaughnessy J, Palumbo A, Durie B et al (2011) Consensus recommendations for risk stratification in multiple myeloma: report of the International Myeloma Workshop Consensus Panel 2. Blood 117(18):4696–4700. https://doi.org/10.1182/blood-2010-10-300970
Saxe D, Seo EJ, Bergeron MB, Han JY (2018) Recent advances in cytogenetic characterization of multiple myeloma. Int J Lab Hematol. https://doi.org/10.1111/ijlh.12882
Kaufmann H, Krömer E, Nösslinger T, Weltermann A, Ackermann J, Reisner R et al (2003) Both chromosome 13 abnormalities by metaphase cytogenetics and deletion of 13q by interphase FISH only are prognostically relevant in multiple myeloma. Eur J Haematol 71(3):179–183
Durak BA, Akay OM, Sungar G, Bademci G, Aslan V, Caferler J et al (2012) Conventional and molecular cytogenetic analyses in Turkish patients with multiple myeloma. Turk J Haematol 29(2):135–142. https://doi.org/10.5152/tjh.2011.42
Huang SY, Yao M, Tang JL, Tsay W, Lee FY, Liu MC et al (2005) Clinical significance of cytogenetics and interphase fluorescence in situ hybridization analysis in newly diagnosed multiple myeloma in Taiwan. Ann Oncol 16(9):1530–1538
Jung HA, Jang MA, Kim K, Kim SH (2018) Clinical utility of a diagnostic approach to detect genetic abnormalities in multiple myeloma: a single institution experience. Ann Lab Med 38(3):196–203. https://doi.org/10.3343/alm.2018.38.3.196
Trcić RL, Skelin IK, Sustercić D, Planinc-Peraica A, Ajduković R, Haris V et al (2010) Cytogenetics of multiple myeloma. Coll Antropol 34(1):41–44
Jekarl DW, Min CK, Kwon A, Kim H, Chae H, Kim M et al (2013) Impact of genetic abnormalities on the prognoses and clinical parameters of patients with multiple myeloma. Ann Lab Med 33(4):248–254
Jian Y, Chen X, Zhou H, Zhu W, Liu N, Geng C et al (2016) Prognostic impact of cytogenetic abnormalities in multiple myeloma: a retrospective analysis of 229 patients. Medicine (Baltimore) 95(19):e3521
Li S, Lim HH, Woo KS, Kim SH, Han JY (2016) A retrospective analysis of cytogenetic alterations in patients with newly diagnosed multiple myeloma: a single center study in Korea. Blood Res 51(2):122–126
Lim AS, Lim TH, See KH, Ng YJ, Tan YM, Choo NS et al (2013) Cytogenetic and molecular aberrations of multiple myeloma patients: a single-center study in Singapore. Chin Med J (Engl) 126(10):1872–1877
Lai YY, Huang XJ, Cai Z, Cao XS, Chen FP, Chen XQ et al (2012) Prognostic power of abnormal cytogenetics for multiple myeloma: a multicenter study in China. Chin Med J (Engl) 125(15):2663–2670
He J, Yang L, Meng X, Wei G, Wu W, Han X et al (2013) A retrospective analysis of cytogenetic and clinical characteristics in patients with multiple myeloma. Am J Med Sci 345(2):88–93
Rack K, Vidrequin S, Dargent JL (2016) Genomic profiling of myeloma: the best approach, a comparison of cytogenetics, FISH and array-CGH of 112 myeloma cases. J Clin Pathol 69(1):82–86
Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C et al (2007) Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myéloma. Blood 8:3489–3495
Fonseca R, Blood E, Rue M, Harrington D, Oken MM, Kyle RA et al (2003) Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood 11:4569–4575
Calasanz MJ, Cigudosa JC, Odero MD, Ferreira C, Ardanaz MT, Fraile A et al (1997) Cytogenetic analysis of 280 patients with multiple myeloma and related disorders: primary breakpoints and clinical correlations. Genes Chromosomes Cancer 18(2):84–93
Mohamed AN, Bentley G, Bonnett ML, Zonder J, Al-Katib A (2007) Chromosome aberrations in a series of 120 multiple myeloma cases with abnormal karyotypes. Am J Hematol 82(12):1080–1087
Sawyer JR, Waldron JA, Jagannath S, Barlogie B (1995) Cytogenetic findings in 200 patients with multiple myeloma. Cancer Genet Cytogenet 82(1):41–49
Lee JW, Lee JK, Hong YJ, Hong SI, Chang YH (2008) Correlation of chromosomal aberrations with prognostic markers in multiple myeloma patients—a single institution studyF. Korean J Lab Med 28(6):413–418
Bang SM, Kim YR, Cho HI, Chi HS, Seo EJ, Park CJ et al (2006) Identification of 13q deletion, trisomy 1q, and IgH rearrangement as the most frequent chromosomal changes found in Korean patients with multiple myeloma. Cancer Genet Cytogenet 168(2):124–132
Chng WJ, Ketterling RP, Fonseca R (2006) Analysis of genetic abnormalities provides insights into genetic evolution of hyperdiploid myeloma. Genes Chromosomes Cancer 45(12):1111–1120
Nilsson T, Höglund M, Lenhoff S, Rylander L, Turesson I, Westin J et al (2003) A pooled analysis of karyotypic patterns, breakpoints and imbalances in 783 cytogenetically abnormal multiple myelomas reveals frequently involved chromosome segments as well as significant age- and sex-related differences. Br J Haematol 120(6):960–969
Brigaudeau C (1999) del(6q) in multiple myeloma. Atlas Genet Cytogenet Oncol Haematol 3(1):17–18
Rajan AM, Rajkumar SV (2015) Interpretation of cytogenetic results in multiple myeloma for clinical practice. Blood Cancer J 5:e365
Bergsagel PL, Nardini E, Brents L, Chesi M, Kuehl WM (1997) IgH translocations in multiple myeloma: a nearly universal event that rarely involves c-myc. Curr Top Microbiol Immunol 224:283–287
Kumar SK, Rajkumar V, Kyle RA, van Duin M, Sonneveld P, Mateos MV et al (2017) Multiple myeloma. Nat Rev Dis Primers 3:17046
Gu G, Sederberg MC, Drachenberg MR, South ST (2014) IGF2BP1: a novel IGH translocation partner in B acute lymphoblastic leukemia. Cancer Genet 207(7–8):332–334
Panani AD (2009) Is there an association with constitutional structural chromosomal abnormalities and hematologic neoplastic process? A short review. Ann Hematol 88(4):293–299
Welborn J (2004) Acquired Robertsonian translocations are not rare events in acute leukemia and lymphoma. Cancer Genet Cytogenet 151(1):14–35
Stone JF, Sandberg AA (1995) Sex chromosome aneuploidy and aging. Mutat Res 338(1–6):107–113
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aydin, C., Ulas, T., Hangul, C. et al. Conventional Cytogenetics and Interphase Fluorescence In Situ Hybridization Results in Multiple Myeloma: A Turkey Laboratory Analysis of 381 Cases. Indian J Hematol Blood Transfus 36, 284–291 (2020). https://doi.org/10.1007/s12288-019-01215-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-019-01215-5