Abstract
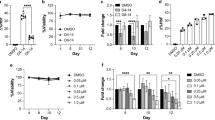
The induction of fetal haemoglobin (Hb F), due to the sustained clinical effects, is one of the most promising methods for the treatment of β hemoglobinopathies, such as thalassemia major and sickle cell disease (SCD). Inhibition of γ-globin gene silencing, possibly is a suitable strategy to induce HbF expression in these patients. In this study, the possibility of increasing HbF in the CD34+ derived erythroid cells was investigated by BCL11A inhibition using specific small-interfering RNAs (siRNAs). Human peripheral blood-derived hematopoietic stem cells were isolated and differentiated to erythroid cells. Erythroid maturation was investigated using cell morphology parameters and flow cytometry analysis of CD235a expression On day 20, siRNA complementary to BCL11A was transfected to differentiating cells via electroporation. BCL11A expression was evaluated through real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) and enzyme linked immunosorbant assay (ELISA). β actin was used as the reference gene to confirm the relative expression level of BCL11A gene mRNA. 48 hours after transfection, BCL11A siRNA significantly reduced BCL11A mRNA levels and consequently led to 2.0 fold decrease in corresponding protein. On the 28th day, haemoglobin electrophoresis results showed that Hb F levels in transfected erythroid cells increased 3.3-fold when compared with non transfected cells. In this study we showed that BCL11A inhibition in erythroid cells could increase fetal hemoglobin, and this strategy can be the basis for designing a γ globin expressing cellular system to increase Hb F in patients with thalassemia and SCD.




Similar content being viewed by others
References
Williams TN, Weatherall DJ (2012) World distribution, population genetics, and health burden of the hemoglobinopathies. Cold Spring Harb Perspect Med 2:a011692
Finotti A, Breda L, Lederer CW, Bianchi N, Zuccato C et al (2015) Recent trends in the gene therapy of β-thalassemia. J Blood Med 6:69–85
Arumugam P, Malik P (2010) Genetic therapy for beta-thalassemia: from the bench to the bedside. Hematol Am Soc Hematol Educ Progr 2010:445–450
Pile FB, Steinberg MH, Rees DC (2017) Sickle cell disease. N Engl J Med 376:1561–1573
Manwani D, Frenette PS (2013) Vaso-occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Blood 122:3892–3898
Lucarelli G, Andreani M, Angelucci E (2002) The cure of thalassemia by bone marrow transplantation. Blood Rev 16(2):81–85
Bank A (2006) Regulation of human fetal hemoglobin: new players, new complexities. Blood 107(2):435–443
Thein SL, Menzel S, Lathrop M et al (2009) Control of fetal hemoglobin: new insights emerging from genomics and clinical implications. Hum Mol Genet 18(R2):R216–R223
Sankaran VG, Xu J, Ragoczy T et al (2009) Developmental and species-divergent globin switching are driven by BCL11A. Nature 460:1093–1097
Menzel S, Garner C, Gut I, Matsuda F, Yamaguchi M, Heath S, Foglio M, Zelenika D, Boland A, Rooks H et al (2007) A QTL influencing F cell production maps to a gene encoding a zinc-finger protein on chromosome 2p15. Nat Genet 39:1197–1199
Sedgewick AE, Timofeev N, Sebastiani P et al (2008) BCL11A is a major HbF quantitative trait locus in three different populations with beta-hemoglobinopathies. Blood Cells Mol Dis 41:255–258
Sankaran VG, Menne TF, Xu J, Akie TE, Lettre G, Van Handel B, Mikkola HK, Hirschhorn JN, Cantor AB, Orkin SH (2008) Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 322:1839–1842
Sankaran VG, Xu J, Byron R et al (2011) A functional element necessary for fetal hemoglobin silencing. N Engl J Med 365(9):807–814
Xu J, Peng C, Sankaran VG, Shao Z, Esrick EB, Chong BG, Ippolito GC, Fujiwara Y, Ebert BL, Tucker PW, Orkin SH (2011) Correction of sickle cell disease in adult mice by interference with fetal hemoglobin silencing. Science 334:993–996
Xu J, Bauer DE, Kerenyi MA, Vo TD, Hou S, Hsu YJ, Yao H, Trowbridge JJ, Mandel G, Orkin SH (2013) Corepressor-dependent silencing of fetal hemoglobin expression by BCL11A. Proc Natl Acad Sci USA 110:6518–6523
Uda M, Galanello R, Sanna S, Lettre G, Sankaran VG, Chen W et al (2008) Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia. Proc Natl Acad Sci USA 105(5):1620–1625
Wilber A, Hargrove PW, Kim YS et al (2011) Therapeutic levels of fetal hemoglobin in erythroid progeny of beta-thalassemic CD34 + cells after lentiviral vector-mediated gene transfer. Blood 117(10):2817–2826
Napoli C, Lemieux C, Jorgensen R (1990) Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in Trans. Plant Cell Online 2(4):279–289
Melnikova I (2007) RNA-based therapies. Nat Rev Drug Discov 6(11):863–864
Skoblov M (2009) Prospects of antisense therapy technologies. Mol Biol 43(6):917–929
Ghosal A, Kabir AH, Mandal A (2011) RNA interference and its therapeutic potential. Cent Euro J Med 6(2):137–147
Fordis CM, Anagnou NP, Dean A, Nienhuis AW, Schechter AN (1984) A beta-globin gene, inactive in the K562 leukemic cell, functions normally in a heterologous expression system. Proc Natl Acad Sci USA 81:4485–4489
Trakarnsanga K, Wilson MC, Lau W et al (2014) Induction of adult levels of beta-globin in human erythroid cells that intrinsically express embryonic or fetal globin by transduction with KLF1 and BCL11A-XL. Haematologica 99:1677–1685
Xu J, Sankaran VG, Ni M et al (2010) Transcriptional silencing of {gamma}-globin by BCL11A involves long-range interactions and cooperation with SOX6. Genes Dev 24(8):783–798
Acknowledgements
This article was extracted from a Ph.D. thesis and was financially supported by Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran. The authors declare no conflict of interest.
Author information
Authors and Affiliations
Contributions
S.A.T., K.M.H.; Contributed to conception and design. S.A.T., K.M.H., GH.T; Contributed to all experimental work and data. S.A.T., K.M.H., L.K.; Contributed to statistical analysis and interpretation of data. K.M.H., GH.T; were responsible for overall supervision. S.A.T.; Drafted the manuscript, which was revised by K.M.H. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors of this article declare that they have no conflict of interest.
Human and Animal Rights
This article does not contain any studies with human participants performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taghavi, S.A., Hosseini, K.M., Tamaddon, G. et al. Inhibition of γ/β Globin Gene Switching in CD 34+ Derived Erythroid Cells by BCL11A RNA Silencing. Indian J Hematol Blood Transfus 35, 758–764 (2019). https://doi.org/10.1007/s12288-019-01131-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-019-01131-8