Abstract
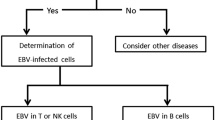
Epstein–Barr virus is the first human oncogenic virus associated with a broad range of different malignant diseases but its role in non-Hodgkin lymphomas (NHL) development still needs to be fully understood. High expression levels of EBV major genes are found in NHL tumor cells and free viral DNA circulates in the plasma of such individuals. In the current study we detected EBV DNA levels in plasma samples from NHL patients in order to validate its significance as a laboratory marker for disease monitoring. We investigated a cohort of 52 patients diagnosed with NHL in The University Hospital “St. Marina” Varna, Bulgaria. Viral DNA was extracted from single plasma samples using Kit Ribo Virus (Sacace Biotechnologies S.r.l., Como, Italy) and amplified with EBV Real-TM Quant (Sacace Biotechnologies S.r.l., Como, Italy). Plasma samples of the same patients were tested for presence of EBV VCA IgM/IgG antibodies with indirect ELISA tests (Euroimmun, Luebeck, Germany). We found 15.4% (95% CI 6.9–28.1%, n = 8) of the samples from NHL patients to be positive in quantitative PCR (range 674–221,333 copies/ml). The diffuse large B cell lymphomas and peripheral T cell lymphomas were most often associated (although not statistically significant, p = 0.167) with detectable plasma EBV DNA levels. To our knowledge, this is the first study about the role of EBV in NHL development in Bulgaria. The results we have obtained should stimulate new, larger investigations to apply the quantitative PCR technique in the routine laboratory EBV diagnosis.


Similar content being viewed by others
References
Ekström-Smedby K (2006) Epidemiology and etiology of non-Hodgkin lymphoma: a review. Acta Oncol (Madr) 45(3):258–271
National Statistical Institute (2018) National center of public health and analyses. Zdraveopazvane, Sofia
Engels EA (2007) Infectious agents as causes of non-Hodgkin lymphoma. Cancer Epidemiol Biomark Prev 16(3):401–404
Cohen JI (2000) Epstein–Barr virus infection. N Engl J Med 343(7):481–492
Sinha M, Rao C, Premalata C, Shafiulla M, Lakshmaiah K, Jacob L et al (2016) Plasma Epstein–Barr virus and Hepatitis B virus in non-Hodgkin lymphomas: two lymphotropic, potentially oncogenic, latently occurring DNA viruses. Indian J Med Paediatr Oncol 37(3):146
Shannon-Lowe CD, Neuhierl B, Baldwin G, Rickinson AB, Delecluse H-J (2006) Resting B cells as a transfer vehicle for Epstein–Barr virus infection of epithelial cells. Proc Natl Acad Sci USA 103(18):7065–7070
Ambinder RF, Mann RB (1994) Detection and characterization of Epstein–Barr virus in clinical specimens. Am J Pathol 145(2):239–252
Hassan R, White LR, Stefanoff CG, de Oliveira DE, Felisbino FE, Klumb CE et al (2006) Epstein–Barr virus (EBV) detection and typing by PCR: a contribution to diagnostic screening of EBV-positive Burkitt’s lymphoma. Diagn Pathol 1:17
Kanakry JA, Li H, Gellert LL, Lemas MV, Hsieh W-S, Hong F et al (2013) Plasma Epstein–Barr virus DNA predicts outcome in advanced Hodgkin lymphoma: correlative analysis from a large North American cooperative group trial. Blood 121(18):3547–3553
An N, Xie Y, Shi P, Xu Y, Qian S (2017) Prognostic significance of circulating plasma Epstein–Barr virus DNA in monitoring aggressive non-Hodgkin’s lymphoma. Int J Clin Exp Med 10(4):6837–6844
Kim SJ, Choi JY, Hyun SH, Ki C-S, Oh D, Ahn YC et al (2015) Risk stratification on the basis of Deauville score on PET-CT and the presence of Epstein–Barr virus DNA after completion of primary treatment for extranodal natural killer/T-cell lymphoma, nasal type: a multicentre, retrospective analysis. Lancet Haematol 2(2):e66–e74
Kim YR, Kim S-J, Cheong J-W, Chung H, Jang JE, Kim Y et al (2017) Pretreatment Epstein–Barr virus DNA in whole blood is a prognostic marker in peripheral T-cell lymphoma. Oncotarget 8(54):92312–92323
Fei Q, Tian X-K, Wu J, Zhu H-M, Wang Y, Peng F-Y et al (2018) Prognostic significance of Epstein–Barr virus DNA in NK/T-cell lymphoma: a meta-analysis. Onco Targets Ther 11:997–1004
Henle W, Henle G, Andersson J, Ernberg I, Klein G, Horwitz CA et al (1987) Antibody responses to Epstein–Barr virus-determined nuclear antigen (EBNA)-1 and EBNA-2 in acute and chronic Epstein–Barr virus infection. Proc Natl Acad Sci USA 84(2):570–574
Bertrand KA, Birmann BM, Chang ET, Spiegelman D, Aster JC, Zhang SM et al (2010) A prospective study of Epstein–Barr virus antibodies and risk of non-Hodgkin lymphoma. Blood 116(18):3547–3553
Teras LR, Rollison DE, Pawlita M, Michel A, Brozy J, de Sanjose S et al (2015) Epstein–Barr virus and risk of non-Hodgkin lymphoma in the cancer prevention study-II and a meta-analysis of serologic studies. Int J Cancer 136(1):108–116
Kostadinova T, Ivanova L, Raykov T, Stojkova Z, Tsankova G (2016) Seroprevalence of Epstein–Barr virus in North-Eastern Bulgaria. Acta Microbiol Bulg 32(3):33–38
Gulley ML, Tang W (2008) Laboratory assays for Epstein–Barr virus-related disease. J Mol Diagn 10(4):279–292
Au W-Y, Pang A, Choy C, Chim C-S, Kwong Y-L (2004) Quantification of circulating Epstein–Barr virus (EBV) DNA in the diagnosis and monitoring of natural killer cell and EBV-positive lymphomas in immunocompetent patients. Blood 104(1):243–249
Ryan JL, Fan H, Glaser SL, Schichman SA, Raab-Traub N, Gulley ML (2004) Epstein–Barr virus quantitation by real-time PCR targeting multiple gene segments. J Mol Diagn 6(4):378–385
Stevens SJ, Pronk I, Middeldorp JM (2001) Toward standardization of Epstein–Barr virus DNA load monitoring: unfractionated whole blood as preferred clinical specimen. J Clin Microbiol 39(4):1211–1216
Chen Y, Zheng X, Chen B, Yang X, Zheng J, Zheng Z et al (2017) The clinical significance of Epstein–Barr virus DNA in peripheral blood mononuclear cells in patients with non-Hodgkin lymphoma. Leuk Lymphoma 58(10):2349–2355
Musacchio JG, Carvalho MDGDC, Morais JCOD, Silva NH, Scheliga A, Romano S et al (2006) Detection of free circulating Epstein–Barr virus DNA in plasma of patients with Hodgkin’s disease. Sao Paulo Med J 124(3):154–157
Kanakry JA, Hegde AM, Durand CM, Massie AB, Greer AE, Ambinder RF et al (2016) The clinical significance of EBV DNA in the plasma and peripheral blood mononuclear cells of patients with or without EBV diseases. Blood 127(16):2007–2017
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare absence of any conflict of interest related to this manuscript.
Financial Support
This project received a Grant No 16003/2016 from the Medical University Varna.
Ethical Approval
The study was approved by the Ethics Committee of the Medical University Varna.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kostadinova, T., Ivanova, L.I., Todorova, T.T. et al. Detection of EBV DNA in Non-Hodgkin Lymphoma Patients in Bulgaria. Indian J Hematol Blood Transfus 35, 465–470 (2019). https://doi.org/10.1007/s12288-019-01088-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-019-01088-8