Abstract
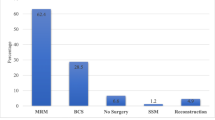
The Japanese Breast Cancer Society initiated the breast cancer registry in 1975, which transitioned to the National Clinical Database-Breast Cancer Registry in 2012. This annual report presents data from 2020 and analyzes the ten-year mortality rates for those aged 65 and older. We analyzed data from 93,784 breast cancer (BC) cases registered in 2020 and assessed 10-year mortality rates for 36,279 elderly patients diagnosed between 2008 and 2012. In 2020, 99.4% of BC cases were females with a median age of 61. Most (65%) were diagnosed at early stages (Stage 0 or I). Breast-conserving surgery rates varied with stages: 58.5% at cStage I, 30.8% at cStage II, and 13.1% at cStage III. Sentinel lymph node biopsy was done in 73.6% of cases, followed by radiotherapy in 70% of those post-conserving surgery and chemotherapy in 21.1% post-surgery. Pathology showed that 63.4% had tumors under 2.0 cm, 11.7% had pTis tumors, and 77.3% had no axillary lymph node metastasis. ER positivity was seen in 75.1%, HER2 in 14.3%, and 30% had a Ki67 positivity rate above 30%. Across all stages and subtypes, there was a trend where the 10-year mortality rates increased for individuals older than 65 years. In Stage I, many deaths were not directly linked to BC and, for those with HER2-type and triple-negative BC, breast cancer-related deaths increased with age. Within Stage II, patients older than 70 years with luminal-type BC often experienced deaths not directly linked to BC, whereas patients below 80 years with HER2-type and triple-negative BC, likely had breast cancer-related deaths. In Stage III, breast cancer-related deaths were more common, particularly in HER2 and triple-negative BC. Our prognostic analysis underscores distinct mortality patterns by stage, subtype, and age in elderly BC patients. It highlights the importance of personalized treatment strategies, considering subtype-specific aggressiveness, age-related factors, and comorbidities.

Similar content being viewed by others
Data availability
The data that support the findings of this study are not openly available due to the nature of the clinical data used. The clinical data are derived from the registry, which is not an open database. The data were accessed by a designated statistician through an application process approved by academic societies. Therefore, we are unable to offer the original clinical data.
References
Kubo M, et al. Annual report of the Japanese Breast Cancer Society Registry for 2016. Breast Cancer. 2020;27(4):511–8.
Hayashi N, et al. Annual report of the Japanese Breast Cancer Registry for 2017. Breast Cancer. 2020;27(5):803–9.
Tada K, et al. Characteristics of female breast cancer in japan: annual report of the National Clinical Database in 2018. Breast Cancer. 2023;30(2):157–66.
Iwamoto T, et al. Distinct breast cancer characteristics between screen- and self-detected breast cancers recorded in the Japanese Breast Cancer Registry. Breast Cancer Res Treat. 2016;156(3):485–94.
Kataoka A, et al. Young adult breast cancer patients have a poor prognosis independent of prognostic clinicopathological factors: a study from the Japanese Breast Cancer Registry. Breast Cancer Res Treat. 2016;160(1):163–72.
Kawai M, et al. Body mass index and survival after diagnosis of invasive breast cancer: a study based on the Japanese National Clinical Database-Breast Cancer Registry. Cancer Med. 2016;5(6):1328–40.
Niikura N, et al. Changes in tumor expression of HER2 and hormone receptors status after neoadjuvant chemotherapy in 21,755 patients from the Japanese breast cancer registry. Ann Oncol. 2016;27(3):480–7.
Kubo M, et al. A population-based recurrence risk management study of patients with pT1 node-negative HER2+ breast cancer: a National Clinical Database study. Breast Cancer Res Treat. 2019;178(3):647–56.
Miyashita M, et al. Role of postmastectomy radiotherapy after neoadjuvant chemotherapy in breast cancer patients: a study from the Japanese Breast Cancer Registry. Ann Surg Oncol. 2019;26(8):2475–85.
Hojo T, et al. Taxane-based combinations as adjuvant chemotherapy for node-positive ER-positive breast cancer based on 2004–2009 data from the Breast Cancer Registry of the Japanese Breast Cancer Society. Breast Cancer. 2020;27(1):85–91.
Ogiya R, et al. Breast cancer survival among Japanese individuals and US residents of Japanese and other origins: a comparative registry-based study. Breast Cancer Res Treat. 2020;184(2):585–96.
Yamada A, et al. Systemic therapy and prognosis of older patients with Stage II/III breast cancer: a large-scale analysis of the Japanese Breast Cancer Registry. Eur J Cancer. 2021;154:157–66.
Aihara T, et al. Prognosis and effectiveness of chemotherapy for medullary breast carcinoma. Breast Cancer Res Treat. 2022;196(3):635–45.
Shimomura A, et al. Clinicopathological features of male patients with breast cancer based on a nationwide registry database in Japan. Breast Cancer. 2022;29(6):985–92.
Terada M, et al. Surgical treatment trends and identification of primary breast tumors after surgery in occult breast cancer: a study based on the Japanese National Clinical Database-Breast Cancer Registry. Breast Cancer. 2022;29(4):698–708.
Yotsumoto D, et al. Trends in adjuvant therapy after breast-conserving surgery for ductal carcinoma in situ of breast: a retrospective cohort study using the National Breast Cancer Registry of Japan. Breast Cancer. 2022;29(1):1–8.
Adachi Y, et al. Analysis of prognosis in different subtypes of invasive lobular carcinoma using the Japanese National Cancer Database-Breast Cancer Registry. Breast Cancer Res Treat. 2023;201(3):397–408.
Acknowledgements
The authors thank all the affiliated institutes participating in the Breast Cancer Registry of the JBCS for their efforts to register the patients’ data.
Funding
This work was funded by the Registration Committee of the Japanese Breast Cancer Society.
Author information
Authors and Affiliations
Contributions
Study concept and design: YS, MM, NN, and TK. Assembly of data: HK and YS. Manuscript writing: YS. Critical revision of the manuscript for important intellectual content: The Registration Committee of the JBCS (NN, YS, TK, TI, NS, KT, MN, NH, MY, CW). Final approval of manuscript: All.
Corresponding author
Ethics declarations
Conflict of interest
YS have received honorariums from Pfizer, Astra Zeneca, Daiichi Sankyo, Eisai, Eli Lily, Chugai Chugai Pharmaceutical Ltd, MSD, and Nihon Kayaku. HK have received honorariums from Chugai Pharmaceutical Ltd and consultation fee from EPS corporation. HK and NK are affiliated to HQA, a social collaborative department supported by National CLinlical Database, Johnson & Johnson KK, Nipro corporation and Intuitive Surgical Sarl. NN have received honorariums from Chugai, Eli Lilly, MSD, Daiichi Sankyo, AstraZeneca, and Pfizer. MM have received honorariums from Chugai, AstraZeneca, Eli Lilly, Pfizer, MSD, Taiho, Daiichi Sankyo and Eisai. TK have received grants from Pfizer Co. Ltd, Kanzawa Medical Research Foundation and Japan Kampo Medicines Manufacturers Association. TI have received grants from Pfizer. MN have received honorariums from AstraZeneca, Eli Lilly, Pfizer, Novartis, Chugai, Taiho, Daiichi Sankyo, Eisai, Kyowa-Kirin, MSD, Myriad genetics, and Denka. NH have received grants from MSD, Chugai, and Konica Minolta Japan and honorariums from Eli Lilly, Astrazeneca, taiho, Eizai, ExactScience, Daiichi-Sankyo, Novartis, Pfizer, and Chugai. MY have received consulting fees from Eli Lilly and Chugai and honorariums from Agilent technologies, Chugai, Ono Yakuhin, MSD and Daiichi Sankyo. CW have received a honorarium from Chugai. MT have received research grants from Chugai, Takeda, Pfizer, Taiho, JBCRG assoc., KBCRN assoc., Eisai, Eli-Lilly and companies, Daiichi-Sankyo, AstraZeneca, Astellas, Shimadzu, Yakult, Nippon Kayaku, AFI technology, Luxonus, Shionogi, GL Science, and Sanwa Shurui. MT have received honorariums from Chugai, Takeda, Pfizer, Kyowa-Kirin, Taiho, Eisai, Daiichi-Sankyo, AstraZeneca, Eli Lilly and companies, MSD, Exact Science, Novartis, Shimadzu, Yakult, Nippon Kayaku, Devicore Medical Japan, and Sysmex. SS have received grants from Taiho, Eisai, Chugai, Takeda, and Daiichi Sankyo and honorariums from Chugai/Roche Astra Zeneca, Eli Lilly, Pfizer, Kyowa Kirin, Daiichi Sankyo and MSD.
Ethical approval
This article does not contain any studies with animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Sagara, Y., Kumamaru, H., Niikura, N. et al. 2020 Annual Report of National Clinical Database-Breast Cancer Registry: 10-year mortality of elderly breast cancer patients in Japan. Breast Cancer 31, 179–184 (2024). https://doi.org/10.1007/s12282-023-01532-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-023-01532-8