Abstract
Background
The aim of this study was to investigate the diagnosis performance of new shear wave elastography (sound touch elastography, STE) in the prediction of neoadjuvant chemotherapy (NAC) response at an early stage in breast cancer patients and to determine the optimal measurement locations around the lesion in different ranges.
Methods
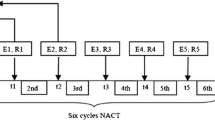
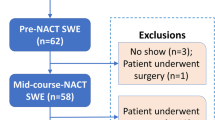
One hundred and eight patients were analyzed in this prospective study from November 2018 to December 2020. All patients completed NAC treatment and underwent STE examination at three time points [the day before NAC (t0); the day before the second course (t1); the day before third course (t2)]. The stiffness of the whole lesion (G), 1-mm shell (S1) and 2-mm shell (S2) around the lesion was expressed by STE parameters. The relative changes (∆stiffness) of STE parameters after the first and second course of NAC were calculated and shown as the variables [Δ(t1) and Δ(t2)]. The diagnostic accuracy of STE was evaluated by means of receiver operating characteristic curve analysis.
Results
The ∆stiffness (%) including ∆Gmean(t2), ∆S1mean(t2) and ∆S2mean(t2) all showed significant differences between pathological complete response (pCR) and non-pCR groups. ∆S2mean(t2) displayed the best predictive performance for pCR (AUC = 0.842) with an ideal ∆stiffness threshold value − 26%.
Conclusions
Measuring the relative changes in the stiffness of surrounding tissue or entire lesion with STE holds promise for effectively predicting the response to NAC at its early stage for breast cancer patients and ∆stiffness of shell 2 mm after the second course of NAC may be a potential prediction parameter.




Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2020;18:452–78.
Brackstone M, Fletcher GG, Dayes IS, Madarnas Y, SenGupta SK, Verma S. Locoregional therapy of locally advanced breast cancer: a clinical practice guideline. Curr Oncol. 2015;22:S54–66.
Baselga J, Campone M, Piccart M, Burris HR, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9.
von Minckwitz G, Blohmer JU, Costa SD, Denkert C, Eidtmann H, Eiermann W, et al. Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2013;31:3623–30.
Bufi E, Belli P, Di Matteo M, Terribile D, Franceschini G, Nardone L, et al. Effect of breast cancer phenotype on diagnostic performance of MRI in the prediction to response to neoadjuvant treatment. Eur J Radiol. 2014;83:1631–8.
Li W, Arasu V, Newitt DC, Jones EF, Wilmes L, Gibbs J, et al. Effect of MR imaging contrast thresholds on prediction of neoadjuvant chemotherapy response in breast cancer subtypes: a subgroup analysis of the ACRIN 6657/I-SPY 1 trial. Tomography. 2016;2:378–87.
Wu J, Gong G, Cui Y, Li R. Intratumor partitioning and texture analysis of dynamic contrast-enhanced (DCE)-MRI identifies relevant tumor subregions to predict pathological response of breast cancer to neoadjuvant chemotherapy. J Magn Reson Imaging. 2016;44:1107–15.
Junker D, De Zordo T, Quentin M, Ladurner M, Bektic J, Horniger W, et al. Real-time elastography of the prostate. Biomed Res Int. 2014;2014:180804.
Faruk T, Islam MK, Arefin S, Haq MZ. The journey of elastography: background, current status, and future possibilities in breast cancer diagnosis. Clin Breast Cancer. 2015;15:313–24.
Bayat M, Denis M, Gregory A, Mehrmohammadi M, Kumar V, Meixner D, et al. Diagnostic features of quantitative comb-push shear elastography for breast lesion differentiation. PLoS One. 2017;12:e172801.
Lee DH, Lee ES, Lee JY, Bae JS, Kim H, Lee KB, et al. Two-dimensional-shear wave elastography with a propagation map: prospective evaluation of liver fibrosis using histopathology as the reference standard. Korean J Radiol. 2020;21:1317–25.
Cantisani V, David E, Grazhdani H, Rubini A, Radzina M, Dietrich CF, et al. Prospective evaluation of semiquantitative strain ratio and quantitative 2D ultrasound shear wave elastography (SWE) in association with TIRADS classification for thyroid nodule characterization. Ultraschall Med. 2019;40:495–503.
Wildeboer RR, Mannaerts CK, van Sloun R, Budaus L, Tilki D, Wijkstra H, et al. Automated multiparametric localization of prostate cancer based on B-mode, shear-wave elastography, and contrast-enhanced ultrasound radiomics. Eur Radiol. 2020;30:806–15.
Cantisani V, David E, Barr RG, Radzina M, de Soccio V, Elia D, et al. US-elastography for breast lesion characterization: prospective comparison of US BIRADS, strain elastography and shear wave elastography. Ultraschall Med. 2021;42:533–40.
Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341–50.
Ulrich TA, de Juan PE, Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009;69:4167–74.
Schrader J, Gordon-Walker TT, Aucott RL, van Deemter M, Quaas A, Walsh S, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53:1192–205.
Corben AD, Abi-Raad R, Popa I, Teo CH, Macklin EA, Koerner FC, et al. Pathologic response and long-term follow-up in breast cancer patients treated with neoadjuvant chemotherapy: a comparison between classifications and their practical application. Arch Pathol Lab Med. 2013;137:1074–82.
Ophir J, Cespedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111–34.
Sandrin L, Tanter M, Catheline S, Fink M. Shear modulus imaging with 2-D transient elastography. IEEE Trans Ultrason Ferroelectr Freq Control. 2002;49:426–35.
Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906.
Zhang J, Tan X, Zhang X, Kang Y, Li J, Ren W, et al. Efficacy of shear-wave elastography versus dynamic optical breast imaging for predicting the pathological response to neoadjuvant chemotherapy in breast cancer. Eur J Radiol. 2020;129:109098.
Yang H, Xu Y, Zhao Y, Yin J, Chen Z, Huang P. The role of tissue elasticity in the differential diagnosis of benign and malignant breast lesions using shear wave elastography. BMC Cancer. 2020;20:930.
Zhou J, Zhan W, Chang C, Zhang X, Jia Y, Dong Y, et al. Breast lesions: evaluation with shear wave elastography, with special emphasis on the “stiff rim” sign. Radiology. 2014;272:63–72.
Maier AM, Heil J, Harcos A, Sinn HP, Rauch G, Uhlmann L, et al. Prediction of pathological complete response in breast cancer patients during neoadjuvant chemotherapy: Is shear wave elastography a useful tool in clinical routine? Eur J Radiol. 2020;128:109025.
Fernandes J, Sannachi L, Tran WT, Koven A, Watkins E, Hadizad F, et al. Monitoring breast cancer response to neoadjuvant chemotherapy using ultrasound strain elastography. Transl Oncol. 2019;12:1177–84.
Zhang X, Liang M, Yang Z, Zheng C, Wu J, Ou B, et al. Deep learning-based radiomics of B-mode ultrasonography and shear-wave elastography: improved performance in breast mass classification. Front Oncol. 2020;10:1621.
Katyan A, Mittal MK, Mani C, Mandal AK. Strain wave elastography in response assessment to neo-adjuvant chemotherapy in patients with locally advanced breast cancer. Br J Radiol. 2019;92:20180515.
Falou O, Sadeghi-Naini A, Prematilake S, Sofroni E, Papanicolau N, Iradji S, et al. Evaluation of neoadjuvant chemotherapy response in women with locally advanced breast cancer using ultrasound elastography. Transl Oncol. 2013;6:17–24.
Ma Y, Zhang S, Li J, Li J, Kang Y, Ren W. Comparison of strain and shear-wave ultrasounic elastography in predicting the pathological response to neoadjuvant chemotherapy in breast cancers. Eur Radiol. 2017;27:2282–91.
Ma Y, Zhang S, Zang L, Li J, Li J, Kang Y, et al. Combination of shear wave elastography and Ki-67 index as a novel predictive modality for the pathological response to neoadjuvant chemotherapy in patients with invasive breast cancer. Eur J Cancer. 2016;69:86–101.
Giussani M, Merlino G, Cappelletti V, Tagliabue E, Daidone MG. Tumor-extracellular matrix interactions: Identification of tools associated with breast cancer progression. Semin Cancer Biol. 2015;35:3–10.
Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat Cell Biol. 2015;17:678–88.
Oskarsson T. Extracellular matrix components in breast cancer progression and metastasis. Breast. 2013;22(Suppl 2):S66–72.
Acknowledgements
This study was supported by the National Key R&D Program of China [2018YFC0114900], the Development Project of National Major Scientific Research Instrument [82027803], the National Natural Science Foundation of China [81971623], Key Project of Natural Science Foundation of Zhejiang Province [LZ20H180001], the Major Research Plan of the National Natural Science Foundation of China [91630311], Zhejiang Society Joint Foundation for Mathematical Medicine [LSY19H180015] and the Natural Science Foundation of Zhejiang Province [SZ20H180002].
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Gu, JH., He, C., Zhao, QY. et al. Usefulness of new shear wave elastography in early predicting the efficacy of neoadjuvant chemotherapy for patients with breast cancer: where and when to measure is optimal?. Breast Cancer 29, 478–486 (2022). https://doi.org/10.1007/s12282-021-01327-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-021-01327-9