Abstract
Background
BRCAness is characterized as the phenotypes shared between some sporadic tumors and BRCA1/2 mutation cancers resulting in defective homologous recombination. The predictive or prognostic value of BRCAness in HER2-negative breast cancer patients who have received neoadjuvant chemotherapy (NAC) is not fully elucidated.
Methods
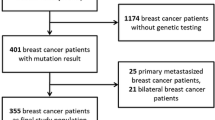
We retrospectively selected 101 high-risk HER2-negative patients diagnosed with stage I–III breast cancer who underwent NAC treatment and evaluated BRCA1-like phenotype using multiplex ligation-dependent probe amplification assay. In an analysis of BRCAness, 95 out of 101 patients were analyzed.
Results
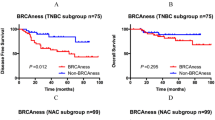
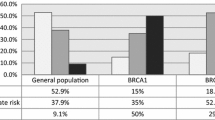
In total, 70 (74%) patients had sporadic-type tumors and 25 (26%) had BRCA1-like tumors according to pre-treatment samples. The BRCA1-like phenotype was not associated with pathological complete response (pCR) rate in the entire cohort. In survival analysis, pre-treatment BRCA1-like phenotype was not associated with survival. On the other hand, post-treatment BRCA1-like patients apparently showed shorter relapse-free survival (log-rank P = 0.016) and breast cancer-specific survival (P < 0.001) compared with sporadic features. In multivariate analysis, only the post-treatment BRCA1-phenotype was significant prognostic factors (HR 5.67, 95% CI 1.19–29.3). Furthermore, we found phenotype change between BRCA1-like and sporadic type through NAC in 19% of non-pCR patients. Post-treatment Ki67 significantly decreased in the persistent sporadic tumors during treatment or sporadic tumors changed after NAC (P < 0.0001, P = 0.0078, respectively).
Conclusions
BRCAness may be useful biomarkers to predict prognosis for HER2-negative breast cancer refractory to standard chemotherapy. Our results pave the way for identifying patients who require alternative therapies.



Similar content being viewed by others
Availability of data and materials
All data generated or analyzed in this study are available from the corresponding author.
Abbreviations
- BCSS:
-
Breast cancer-specific survival
- CI:
-
Confidential interval
- ER:
-
Estrogen receptor
- HER2:
-
Human epidermal growth factor receptor-2
- HR:
-
Hazard ratio
- MLPA:
-
Multiplex ligation-dependent probe amplification
- NAC:
-
Neoadjuvant chemotherapy
- OS:
-
Overall survival
- pCR:
-
Pathological complete response
- PARP:
-
Poly ADP-ribose polymerase
- PR:
-
Progesterone receptor
- RFS:
-
Relapse-free survival
- TNBC:
-
Triple-negative breast cancer
References
Easton DF, Pharoah PD, Antoniou AC, Tischkowitz M, Tavtigian SV, Nathanson KL, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372:2243–57.
Momozawa Y, Iwasaki Y, Parsons MT, Kamatani Y, Takahashi A, Tamura C, et al. Germline pathogenic variants of 11 breast cancer genes in 7,051 Japanese patients and 11,241 controls. Nat Commun. 2018;9:4083.
Cerbinskaite A, Mukhopadhyay A, Plummer ER, Curtin NJ, Edmondson RJ. Defective homologous recombination in human cancers. Cancer Treat Rev. 2012;38:89–100.
Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402–16.
Foulkes WD, Chappuis PO, Wong N, Brunet JS, Vesprini D, Rozen F, et al. Primary node negative breast cancer in BRCA1 mutation carriers has a poor outcome. Ann Oncol. 2000;11:307–13.
Zhong Q, Peng HL, Zhao X, Zhang L, Hwang WT. Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: a meta-analysis. Clin Cancer Res. 2015;21:211–20.
Huzarski T, Byrski T, Gronwald J, Gorski B, Domagala P, Cybulski C, et al. Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J Clin Oncol. 2013;31:3191–6.
Goodwin PJ, Phillips KA, West DW, Ennis M, Hopper JL, John EM, et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population-based cohort study. J Clin Oncol. 2012;30:19–26.
Lancaster JM, Wooster R, Mangion J, Phelan CM, Cochran C, Gumbs C, et al. BRCA2 mutations in primary breast and ovarian cancers. Nat Genet. 1996;13:238–40.
Turner N, Tutt A, Ashworth A. Hallmarks of “BRCAness” in sporadic cancers. Nat Rev Cancer. 2004;4:814–9.
Mori H, Kubo M, Kai M, Velasquez VV, Kurata K, Yamada M, et al. BRCAness combined with a family history of cancer is associated with a poor prognosis for breast cancer patients with a high risk of BRCA mutations. Clin Breast Cancer. 2018;18:e1217–27.
Liu L, Matsunaga Y, Tsurutani J, Akashi-Tanaka S, Masuda H, Ide Y, et al. BRCAness as a prognostic indicator in patients with early breast cancer. Sci Rep. 2020;10:21173.
Das M. Olaparib provides benefit in metastatic breast cancer. Lancet Oncol. 2017;18:e376.
Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–33.
Vollebergh MA, Lips EH, Nederlof PM, Wessels LFA, Schmidt MK, van Beers EH, et al. An aCGH classifier derived from BRCA1-mutated breast cancer and benefit of high-dose platinum-based chemotherapy in HER2-negative breast cancer patients. Ann Oncol. 2011;22:1561–70.
Schouten PC, Marme F, Aulmann S, Sinn HP, van Essen HF, Ylstra B, et al. Breast cancers with a BRCA1-like DNA copy number profile recur less often than expected after high-dose alkylating chemotherapy. Clin Cancer Res. 2015;21:763–70.
Severson TM, Wolf DM, Yau C, Peeters J, Wehkam D, Schouten PC, et al. The BRCA1ness signature is associated significantly with response to PARP inhibitor treatment versus control in the I-SPY 2 randomized neoadjuvant setting. Breast Cancer Res. 2017;19:99.
Kosaka Y, Yamamoto Y, Tanino H, Nishimiya H, Yamamoto-Ibusuki M, Hirota Y, et al. BRCAness as an important prognostic marker in patients with triple-negative breast cancer treated with neoadjuvant chemotherapy: a multicenter retrospective study. Diagnostics (Basel). 2020;10:119.
Lips EH, Laddach N, Savola SP, Vollebergh MA, Oonk AM, Imholz AL, et al. Quantitative copy number analysis by Multiplex Ligation-dependent Probe Amplification (MLPA) of BRCA1-associated breast cancer regions identifies BRCAness. Breast Cancer Res. 2011;13:R107.
Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147–59.
Mamounas EP, Untch M, Mano MS, Huang CS, Geyer CE Jr, von Minckwitz G, et al. Adjuvant T-DM1 versus trastuzumab in patients with residual invasive disease after neoadjuvant therapy for HER2-positive breast cancer: subgroup analyses from KATHERINE. Ann Oncol. 2021;32:1005–14.
Telli ML, Timms KM, Reid J, Hennessy B, Mills GB, Jensen KC, et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin Cancer Res. 2016;22:3764–73.
Belli C, Duso BA, Ferraro E, Curigliano G. Homologous recombination deficiency in triple negative breast cancer. Breast. 2019;45:15–21.
Gross E, van Tinteren H, Li Z, Raab S, Meul C, Avril S, et al. Identification of BRCA1-like triple-negative breast cancers by quantitative multiplex-ligation-dependent probe amplification (MLPA) analysis of BRCA1-associated chromosomal regions: a validation study. BMC Cancer. 2016;16:811.
Tian T, Shan L, Yang W, Zhou X, Shui R. Evaluation of the BRCAness phenotype and its correlations with clinicopathological features in triple-negative breast cancers. Hum Pathol. 2019;84:231–8.
Wang Y, Ung MH, Cantor S, Cheng C. Computational investigation of homologous recombination DNA repair deficiency in sporadic breast cancer. Sci Rep. 2017;7:15742.
Chen Y, Wang Y, Salas LA, Miller TW, Mark K, Marotti JD, et al. Molecular and epigenetic profiles of BRCA1-like hormone-receptor-positive breast tumors identified with development and application of a copy-number-based classifier. Breast Cancer Res. 2019;21:14.
Lips EH, Benard-Slagter A, Opdam M, Scheerman CE, Wesseling J, Hogervorst FBL, et al. BRCAness digitalMLPA profiling predicts benefit of intensified platinum-based chemotherapy in triple-negative and luminal-type breast cancer. Breast Cancer Res. 2020;22:79.
Schroth W, Buttner FA, Kandabarau S, Hoppe R, Fritz P, Kumbrink J, et al. Gene expression signatures of BRCAness and tumor inflammation define subgroups of early-stage hormone receptor-positive breast cancer patients. Clin Cancer Res. 2020;26:6523–34.
Weigelt B, Geyer FC, Reis-Filho JS. Histological types of breast cancer: how special are they? Mol Oncol. 2010;4:192–208.
Stingl J, Caldas C. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat Rev Cancer. 2007;7:791–9.
Keenan TE, Guerriero JL, Barroso-Sousa R, Li T, O’Meara T, Giobbie-Hurder A, et al. Molecular correlates of response to eribulin and pembrolizumab in hormone receptor-positive metastatic breast cancer. Nat Commun. 2021;12:5563.
Liu D, Schilling B, Liu D, Sucker A, Livingstone E, Jerby-Arnon L, et al. Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat Med. 2019;25:1916–27.
Liu D, Abbosh P, Keliher D, Reardon B, Miao D, Mouw K, et al. Mutational patterns in chemotherapy resistant muscle-invasive bladder cancer. Nat Commun. 2017;8:2193.
von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–28.
Watkins JA, Irshad S, Grigoriadis A, Tutt AN. Genomic scars as biomarkers of homologous recombination deficiency and drug response in breast and ovarian cancers. Breast Cancer Res. 2014;16:211.
Muggia F, Safra T. “BRCAness” and its implications for platinum action in gynecologic cancer. Anticancer Res. 2014;34:551–6.
Acknowledgements
The authors are grateful to I. Suzu and H. Moriyama for excellent technical support, and to M. Kawakami for clinical data management.
Author information
Authors and Affiliations
Contributions
AS contributed to the conception and design of the study, performed experiments, and wrote the manuscript. MYI contributed to performing experiments and analysis of raw data. YY participated in the study design and coordination and helped to draft the manuscript. HI participated in study design. LGY, YY, and MT helped to draft the manuscript. All authors reviewed and approved the submitted manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Y. Yamamoto has relevant conflicts of interest, as follows: personal fees from Chugai, AstraZeneca, Kyowa-Kirin, Pfizer, Novartis, Essai, Takeda, Taiho, GE Health Care Japan, Nippon Kayaku, Daiichi-Sankyo, Sysmex, and Lilly; grants from Chugai, Pfizer, AstraZeneca, Kyowa-Kirin, Daiichi-Sankyo, Nippon Kayaku, and Lilly.
The other authors have no conflicts of interest to declare.
Ethical approval
The entire study was approved by the ethics committee of Kumamoto University Graduate School of Medical Sciences.
Consent to participate
Written informed consent was obtained from all subjects for the collection and research use of serum samples. All relevant methods were performed according to the relevant guidelines and regulations.
Patients consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Sueta, A., Yamamoto-Ibusuki, M., Tomiguchi, M. et al. Predictive and prognostic significance of BRCAness in HER2-negative breast cancer. Breast Cancer 29, 368–376 (2022). https://doi.org/10.1007/s12282-021-01319-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-021-01319-9