Abstract
Background
Although there are a large number of epidemiological studies investigating the etiological role of lifestyle factors in breast cancer, there are few studies on the association between lifestyle factors and breast cancer prognosis. To investigate the influence of lifestyle factors such as diet and physical activity, use of complementary and alternative medicine, and psychosocial factors on prognosis, we designed a large-scale cohort study of female breast cancer patients in Japan.
Methods
The planned sample size is 7200. The cohort is being conducted in collaboration with several clinical trials, a cancer registry, and daily practice. Information on clinical factors, treatment, and follow-up will be obtained from the clinical trials and participating hospitals. A self-administered questionnaire is given to subjects before, immediately after, or 1 to 5 years after surgery. Blood and tissue samples are also collected. The primary endpoint is disease-free survival. The secondary endpoints are overall survival and health-related quality of life. The follow-up period will be at least 5 years after the last participant is enrolled. Recruitment began in November 2007.
Current status
As of April 2017, there are 5852 patients enrolled in the study along with 1430 biological samples and the study is still ongoing. The number of subjects enrolled in the study is already the largest in the world.
Conclusions
The ROK study will provide much important evidence for breast cancer survivorship.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a growing number of cancer survivors in the world. Especially, since the prognosis of breast cancer is relatively favorable compared to other types of cancer [1], there are a large number of breast cancer survivors. In addition to medical treatments, patients are also willing to make their own efforts to prevent recurrence, such as changing their diet and levels of physical activity and incorporating complementary and alternative medicine (CAM) into their daily lives. Although there is growing number of epidemiological studies investigating the etiological role of lifestyle factors in breast cancer, many fewer studies have examined the association between modifiable risk factors such as lifestyle and CAM use and breast cancer recurrence and survival [2, 3]. Recently, several prospective cohort studies have been designed and are being conducted [4, 5], but as the studies have just started, sufficient evidence has not been obtained. Such information is eagerly awaited by breast cancer patients as well as breast cancer researchers.
In addition to these factors, psychosocial factors play important roles on patient’s quality of life (QOL). For this purpose, the association between lifestyle and psychosocial factors and prognosis should be studied, and appropriate information must be provided to patients, their families, and health professionals.
In this ROK (Rainbow of KIBOU) study, a large cohort of breast cancer patients is being conducted to examine the influence of lifestyle factors, CAM use, psychosocial factors, pain, and supportive care on prognosis such as QOL, recurrence, and survival. Furthermore, in part of the cohort, blood samples and tissues are being collected to examine the association between prognosis and several biomarkers such as serum nutrient levels and genetic polymorphisms, and somatic mutations.
Methods
Study structure
The ROK study consists of several cohorts (Table 1). Some of the cohorts are designed as part of a collaborative study with several multi-institutional randomized control trials involving female breast cancer patients. A self-administered questionnaire is delivered to patients enrolled in these trials, and their responses are regarded as baseline data of the cohort. Follow-up and clinical information will be obtained from the participating institutions. We are conducting 3 cohorts in collaboration with clinical trials: cohort 05, cohort 06, and cohort 07. Cohort 05 is a collaborative study with the National Surgical Adjuvant Study of Breast Cancer (N-SAS BC) 05; Randomized Study to Assess the Efficacy of a Further 5 Years of Anastrozole Treatment for Postmenopausal Women with Breast Cancer Completing 5 Years of Anastrozole Containing Adjuvant Endocrine Therapy (AERAS). Cohort 06 is a collaborative study with the N-SAS BC06; Randomized Phase III Study of Adjuvant Endocrine Therapy with or without Chemotherapy for Postmenopausal Breast Cancer Patients who Responded to Neoadjuvant Letrozole (NEOS). Cohort 07 is a collaborative study with N-SAS BC07; Evaluation of Trastuzumab without Chemotherapy as a Postoperative Adjuvant Therapy in HER2-positive Elderly Breast Cancer Patients: Randomized Controlled Trial (RESPECT). These trials are directed by the Comprehensive Support Project for Oncology Research (CSPOR) of the Public Health Research Foundation, Tokyo, Japan.
In addition to the cohorts associated with these clinical trials, other cohorts are being recruited at the National Cancer Center Hospital in Tokyo, Japan (cohort NCC) and a collaborative study with the Setouchi Cancer Registry (cohort Setouchi). Blood and tissue samples are being collected in cohort NCC in addition to questionnaire data.
Participants of the study
Eligibility criteria for cohorts 05, 06, and 07 are based on the eligibility of the respective clinical trial. Patients who give informed consent for participation in the trials are considered eligible for the cohort study. The eligible subjects for the N-SAS BC05 study are postmenopausal women with hormone-responsive primary breast cancer aged less than 80 years. The eligible subjects for the N-SAS BC06 study are postmenopausal women with primary breast cancer aged less than 75 years [6]. The eligible subjects for the N-SAS BC07 study are women with HER2-positive primary breast cancer aged between 70 and 80 years [7]. Details are available at http://www.umin.ac.jp/ctr/ (Protocol ID: UMIN000000818 for N-SAS BC05, UMIN000001090 for N-SAS BC06, and UMIN000002349 for N-SAS BC07). For cohorts NCC and Setouchi, the eligibility criteria include females with primary breast cancer, age 20 years or older, anticipating surgery at the National Cancer Center Hospital or institutions participating in the Setouchi Cancer Registry. Among patients meeting the eligibility criteria, those who provide informed consent for participation in this study are considered eligible.
Survey items and measurements
Questions regarding lifestyle factors are based on the questionnaire used in the Japan Public Health Center-based Prospective Study (JPHC study) [8]. In Europe and the United States, cohort studies have been conducted to evaluate the influence of lifestyle factors such as diet and physical activity, as well as obesity, on recurrence in breast cancer patients [2, 3]. Unfortunately, there are still very few such studies, and sufficient evidence has not been obtained.
In addition to diet and exercise, many patients are interested in and actively practice CAM. This includes the use of oral and topical agents and health-promoting methods such as acupuncture, moxibustion, and yoga, which differ from health insurance-supported practice. Although approximately 50% of cancer patients utilize CAM in Japan [9], there is insufficient evidence regarding its efficacy [10]. CAM utilization is assessed with items based on previous studies [9].
Psychosocial issues reported in breast cancer patients include depression and hopelessness, escape-avoidance coping styles, and stress related to socioeconomic changes [11, 12]. Some previous studies have reported an association between hopelessness, coping style, and recurrence, whereas others have found no association [13, 14]. Therefore, in clinical practice, strategies to manage these psychosocial issues tend to be actively pursued. To evaluate psychological wellbeing, we employ the Herth Hope Index (HHI) [15]. To assess depression, the Center for Epidemiologic Studies Depression Scale (CES-D) [16] is used.
In cohort NCC, blood is collected from the participant. Using plasma or serum samples, the following is the list of candidate substances to be measured: endogenous hormones, insulin and insulin-like growth factor, adipocytokines, markers of inflammation, nutrients, and other substances.
The following is the list of candidates genes to be assessed using DNA samples: genetic polymorphisms in hormone synthesized and metabolized genes, hormone receptor genes, genes related to insulin-like growth factor, genes related to one carbon metabolism, vitamin D receptor genes, genes related to oxidative stress, and genes related to the metabolism of therapeutic agents.
Endpoint
The primary endpoint is disease-free survival. The secondary endpoints are overall survival and health-related quality of life (HRQOL). HRQOL is measured using the Medical Outcome Study-Short Form 36 (SF-36) [17], EuroQol 5 Dimensions [18], Hospital Anxiety and Depression Scale [19], CES-D, Functional Assessment of Cancer Therapy(FACT)-General [20], FACT-Breast [21], FACT-Endocrine Symptoms [22], FACT-Taxane [23], Patient Neurotoxicity Questionnaire [24], Mini-Mental State Examination [25], and Philadelphia Geriatric Center Moral Scale [26].
Data collection
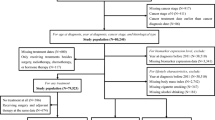
The timing of survey administration during the disease course is shown in Fig. 1. For cohorts 05, 06, and 07, upon registration in the clinical trial, attending physicians explained the purpose and contents of this study, and handed out a self-administered questionnaire to the subjects. They completed the questionnaire at home and returned it by mail. The questionnaire survey was administered once to each participant in cohort 05. In cohort 06, the questionnaire was administered upon primary registration into the clinical trial before surgery, within 8 weeks after surgery, and 12 months after the start of the postoperative treatment protocol for a total of 3 times. In cohort 07, the questionnaire was administered on primary registration into the clinical trial after surgery and 1 year after the start of the postoperative protocol treatment.
In cohorts NCC and Setouchi, the questionnaire is administered upon primary registration into the study before surgery and each year after surgery for 5 years for a total of 5 or 6 times. For each survey, attending physicians explain details of this study, and hand out a questionnaire. In cohort NCC, blood sampling is also conducted at the same time as the questionnaire.
Sample size and study period
The target number of registered patients is 7200 in total. The cohort 05, 06, and 07 finished their registration and the cohort NCC and Setouchi are still enrolling patients. The follow-up period will last for 5 years after the last participant has been enrolled, except for cohort 07, in which patients will be followed 3 years after the last patient was enrolled. The association between the number of expected events and the hypothesis being investigated is shown in Table 2.
Statistical methods
The association between questionnaire items and subsequent prognosis will be examined. The data from the clinical trials is managed at the CSPOR data center and the questionnaire data is managed at the epidemiologic data center for the cohort study. The effect of factors of interest on outcomes will be examined using regression models that adjust for the effects of potential confounders. The models to be used (Cox, logistic, and linear, etc.) will be chosen based on the type of outcome variable.
Subcohorts will be analyzed separately and also analyzed as a combined cohort. As shown in Fig. 1, several subcohorts have collected data on the same timing. For example, cohort 06, 07, NCC and Setouchi collected lifestyle data before surgery and 1 year after surgery. These data are analyzed as a baseline data compared with prognosis. As for cohort 05, NCC and Setouchi, they collected data 5 years after surgery and these data can be analyzed for the association with the prognosis after 5 years. In principle, as for the combination of all the cohorts with different timing from surgery, we plan to analyze the data with meta-analytic approach. Meta-analytic approach can be justified when the effect of interest are the same across cohorts.
Ethical approval
A protocol was prepared for each subcohort and approved by the Institutional Review Board of each participating institution. All investigators participating in this study are compliant with the guidelines for research ethics in Japan and the Helsinki Declaration.
Current status
In total, there are already 5852 subjects enrolled as of April 30, 2017 (Table 2). The registration of cohort 05, 06, 07 successfully ended with 1510, 735, and 311 patients enrolled respectively. The participation rates are surprisingly high with more than 95%. The registration of cohort NCC and Setouchi are still ongoing with already 1592 and 1704 patients enrolled.
Table 3 shows selected baseline characteristics of the ROK study based for already closed cohort 05, 06, and 07. These collaborative cohorts with the clinical trials have a limited age range due to the eligibility criteria of the trials. Patients are enrolled from institutions all over Japan. About 70% of the subjects stated they are “married,” but others answered that they have no husbands for cohort 05 and 06. In cohort 07, the proportion is “married” is much lower since it has a much older age distribution. As for the questions concerning current employment, about 60% of the subjects replied that they are housewives or unemployed in cohorts 05 and 06, and 75% in cohort 07. Regarding CES-D, more than 30% revealed a tendency towards mild (16 ≤ score ≤ 26) or severe depression (score ≥27) when they were asked after breast cancer diagnosis (cohort 06, 07) but the proportion of mild and severe depression was much lower (19%) when CES-D was administered 5 years after diagnosis (cohort 05). More than 95% of each cohort endorsed that they have had at least one perceived positive change after the diagnosis of breast cancer and the number of perceived positive changes tended to be larger in cohort 05, for whom data was collected 5 years after diagnosis. The HHI distribution did not vary substantially across cohorts.
Special feature of the ROK study
The ROK study is one of the first comprehensive prospective cohort studies investigating the influence of lifestyle factors, use of CAM, psychosocial factors, pain, and supportive care on prognosis, including QOL, recurrence, and death in patients with breast cancer. The ROK study has already enrolled more than 5800 subjects and is one of the largest breast cancer cohort studies in the world. Participation rates are greater than 90% and approximately 65 participants are recruited each month. Compared with other patients cohort [2,3,4,5], the ROK study is the second Asian [27] and the first Japanese cohort and only cohorts who includes long time survivor at least 5 years post-diagnosis. We are planning to enroll patients for another 2 years to achieve the target sample size. Since the follow-up periods of the cohort 05 and the cohort 07 will ends in 2017, the first result can be appeared in near future. The combined results of all the cohorts will also be gradually published.
The strengths of the ROK study include having subcohorts as collaborations with clinical trials. If it were in observational studies, treatment is one of the strongest confounders, but this confounding effect is almost perfectly controlled in randomized controlled trials. In addition, data on other potential confounders related to clinical factors are precisely collected in clinical trials. Recurrence can be used as an endpoint in the ROK study because a valid and reproducible definition is used to confirm the date of recurrence in clinical trials, whereas most observational cohort studies can only use death as an endpoint. Another strength is that many hypotheses can be investigated in the ROK study since it collects data before diagnosis, after surgery, and every year after surgery until the 5th year in the same subcohorts. Since institutions from all over Japan are participating in the study, there is a diverse variability in exposure and generalizable results will be obtained. Since blood and tissue data are collected for cohort NCC, results from the questionnaire data can be validated using biomarkers, for example, for the association between certain nutrients and recurrence. Gene-environment interactions can be investigated and subtype specific analysis based on the gene profile can be also conducted.
In addition to the lifestyle factors, the ROK study focuses on psychosocial factors. One of the main objectives of the ROK study is to investigate the psychosocial factors influencing survivorship, including the HHI, perceived positive change due to cancer, ikigai (source of joy and reasons for living), job, social activities, and support that are important to living with breast cancer. This study will clarify the importance of these factors in terms of their association with prognosis and long term QOL. It will provide evidence-based guidance about how to improve prognosis and QOL. There are almost no cohort studies that collect data on these factors.
In summary, the ROK study is one of the largest cohort studies investigating the effect of lifestyle, CAM use, and psychosocial factors on the prognosis of breast cancer. It includes one subcohort with blood and tissue sampling so that various hypotheses can be investigated. So far, the effect of these modifiable factors by patients is not known and this information is eagerly awaited by patients. The ROK study will yield important evidence in the near future.
References
Center for Cancer Control and Information Services, National Cancer Center, Japan. Monitoring of Cancer Incidence in Japan—Survival 2003–2005 Report. 2013. http://ganjoho.jp/data/professional/statistics/odjrh3000000hwsa-att/cancer_survival(1993-2005).xls.
Caan B, Sternfeld B, Gunderson E, Coates A, Quesenberry C, Slattery ML. Life After Cancer Epidemiology (LACE) Study: a cohort of early stage breast cancer survivors (United States). Cancer Causes Control. 2005;16:545–56.
Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, et al. Physical activity levels before and after a diagnosis of breast carcinoma. The Health, Eating, Activity, and Lifestyle (HEAL) Study. Cancer. 2003;97:1746–57.
Kwan ML, Ambrosone CB, Lee MM, Barlow J, Krathwohl SE, Ergas IJ, et al. The Pathways Study: a prospective study of breast cancer survivorship within Kaiser Permanente Northern California. Cancer Causes Control. 2008;19:1065–76.
Swann R, Perkins KA, Velentzis LS, Ciria C, Dutton SJ, Mulligan AA, et al. The DietCompLyf study: a prospective cohort study of breast cancer survival and phytoestrogen consumption. Maturitas. 2013;75:232–40.
Iwata H. Neoadjuvant endocrine therapy for postmenopausal patients with hormone receptor-positive early breast cancer: a new concept. Breast Cancer. 2011;18(2):92–7.
Sawaki M, Tokudome N, Mizuno T, Nakayama T, Taira N, Bando H, et al. Evaluation of trastuzumab without chemotherapy as a post-operative adjuvant therapy in HER2-positive elderly breast cancer patients: randomized controlled trial [RESPECT (N-SAS BC07)]. Jpn J Clin Oncol. 2011;41:709–12.
Tsugane S, Kobayashi M, Sasaki S, JPHC. Validity of the self-administered food frequency questionnaire used in the 5-year follow-up survey of the JPHC Study Cohort I: comparison with dietary records for main nutrients. J Epidemiol. 2003;13:S51–6.
Hyodo I, Amano N, Eguchi K, Narabayashi M, Imanishi J, Hirai M, et al. Nationwide survey on complementary and alternative medicine in cancer patients in Japan. J Clin Oncol. 2005;23:2645–54.
Ernst E, Schmidt K, Baum M. Complementary/alternative therapies for the treatment of breast cancer. A systematic review of randomized clinical trials and a critique of current terminology. Breast J. 2006;12:526–30.
Knobf MT. Psychosocial responses in breast cancer survivors. Semin Oncol Nurs. 2007;23:71–83.
Kornblith AB, Ligibel J. Psychosocial and sexual functioning of survivors of breast cancer. Semin Oncol. 2003;30:799–813.
Watson M, Homewood J, Haviland J, Bliss JM. Influence of psychological response on breast cancer survival: 10-year follow-up of a population-based cohort. Eur J Cancer. 2005;41:1710–4.
Nielsen NR, Grønbæk M. Stress and breast cancer: a systematic update on the current knowledge. Nat Clin Pract Oncol. 2006;3:612–20.
Herth K. Abbreviated instrument to measure hope: development and psychometric evaluation. J Adv Nurs. 1992;17:1251–9.
Radloff LS. The CES-D Scale: a self-report depression scale for research in the general Population. Appl Psychol Meas. 1977;11:385–401.
Ware JE, Kosinski M, Dewey JE. How to score version two of the SF-36 health survey. Lincoln: QualityMetric, Incorporated; 2001.
Brooks R, EuroQol Group. EuroQol: the current state of play. Health Policy. 1996;37:58–72.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70.
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–9.
Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15:974–86.
Fallowfield LJ, Leaity SK, Howell A, Cella D. Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat. 1999;55:189–99.
Cella D, Peternan A, Hudgens S, Webster K, Socinski MA. Measuring the side effects of taxane therapy in oncology. The functional assessment of cancer therapy-taxane (FACT-Taxane). Cancer. 2003;98:822–31.
Kawahara M, Tada H, Tokoro A, Teramukai S, Origasa H, Kubota K, et al. Quality-of-life evaluation for advanced non-small-cell lung cancer: a comparison between vinorelbine plus gemcitabine followed by docetaxel versus paclitaxel plus carboplatin regimens in a randomized trial: Japan Multinational Trial Organization LC00-03 (BRI LC03-01). BMC Cancer. 2011;11:356.
Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
Lawton MP. The Philadelphia Geriatric Center Morale Scale: a revision. J Gerontol. 1975;30:85–9.
Shu XO, Zheng Y, Cai H, Gu K, Chen Z, Zheng W, Lu W. Soy food intake and breast cancer survival. JAMA. 2009;302(22):2437–43. doi:10.1001/jama.2009.1783.
Acknowledgements
We greatly appreciate the cooperation of the participating institutions. We would also like to express sincere appreciation to all the women who participated in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by grants from the Ministry of Health, Labour and Welfare of Japan and the research and development funds of the National Cancer Center (25-A-16, 28-A-20). Funding sources have no involvement in any aspects of the study.
Conflict of interest
The authors have no conflict of interest.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Mizota, Y., Ohashi, Y., Iwase, T. et al. Rainbow of KIBOU (ROK) study: a Breast Cancer Survivor Cohort in Japan. Breast Cancer 25, 60–67 (2018). https://doi.org/10.1007/s12282-017-0784-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-017-0784-x