Abstract
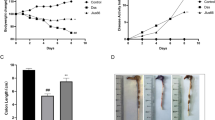
Although phosphatase and tensin homolog (PTEN) is typically considered a tumor-suppressor gene, it was recently suggested that PTEN regulates TLR5-induced immune and inflammatory responses in intestinal epithelial cells (IECs), suggesting an immunomodulatory function of PTEN in the gut. However, this alternative function of PTEN has not yet been evaluated in an in vivo context of protection against enteropathogenic bacteria. To address this, we utilized IEC-restricted Pten knockout (PtenΔIEC/ΔIEC) and littermate Pten+/+ mice. These mice were subjected to the streptomycin-pre-treated mouse model of Salmonella infection, and subsequently given an oral gavage of a low inoculum (2 × 104 CFU) of Salmonella enterica serovar Typhimurium (S. Typhimurium). This bacterial infection not only increased the mortality of PtenΔIEC/ΔIEC mice compared to Pten+/+ mice, but also induced deleterious gastrointestinal inflammation in PtenΔIEC/ΔIEC mice manifested by massive histological damage to the intestinal mucosa. S. Typhimurium infection up-regulated pro-inflammatory cytokine production in the intestine of PtenΔIEC/ΔIEC mice compared to controls. Furthermore, bacterial loads were greatly increased in the liver, mesenteric lymph node, and spleen of PtenΔIEC/ΔIEC mice compared to controls. Together, these results suggest that IEC-restricted Pten deficiency renders the host greatly susceptible to Salmonella infection and support an immune-regulatory role of PTEN in the gut.
Similar content being viewed by others
References
Altmeyer, M., Barthel, M., Eberhard, M., Rehrauer, H., Hardt, W.D., and Hottiger, M.O. 2010. Absence of poly(ADP-ribose) polymerase 1 delays the onset of Salmonella enterica serovar Typhimurium-induced gut inflammation. Infect. Immun. 78, 3420–3431.
Barthel, M., Hapfelmeier, S., Quintanilla-Martinez, L., Kremer, M., Rohde, M., Hogardt, M., Pfeffer, K., Russmann, H., and Hardt, W.D. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 71, 2839–2858.
Bravo-Blas, A., Utriainen, L., Clay, S.L., Kastele, V., Cerovic, V., Cunningham, A.F., Henderson, I.R., Wall, D.M., and Milling, S.W.F. 2019. Salmonella enterica serovar Typhimurium travels to mesenteric lymph nodes both with host cells and autonomously. J. Immunol. 202, 260–267.
Cao, X., Wei, G., Fang, H., Guo, J., Weinstein, M., Marsh, C.B., Ostrowski, M.C., and Tridandapani, S. 2004. The inositol 3-phosphatase PTEN negatively regulates Fcγ receptor signaling, but supports toll-like receptor 4 signaling in murine peritoneal macrophages. J. Immunol. 172, 4851–4857.
Choi, Y.J., Im, E., Chung, H.K., Pothoulakis, C., and Rhee, S.H. 2010. TRIF mediates toll-like receptor 5-induced signaling in intestinal epithelial cells. J. Biol. Chem. 285, 37570–37578.
Choi, Y.J., Jung, J., Chung, H.K., Im, E., and Rhee, S.H. 2013. PTEN regulates TLR5-induced intestinal inflammation by controlling Mal/TIRAP recruitment. FASEB J. 27, 243–254.
Ellenbroek, G.H., van Puijvelde, G.H., Anas, A.A., Bot, M., Asbach, M., Schoneveld, A., van Santbrink, P.J., Foks, A.C., Timmers, L., Doevendans, P.A., et al. 2017. Leukocyte TLR5 deficiency inhibits atherosclerosis by reduced macrophage recruitment and defective T-cell responsiveness. Sci. Rep. 7, 42688.
Erova, T.E., Kirtley, M.L., Fitts, E.C., Ponnusamy, D., Baze, W.B., Andersson, J.A., Cong, Y., Tiner, B.L., Sha, J., and Chopra, A.K. 2016. Protective immunity elicited by oral immunization of mice with Salmonella enterica serovar Typhimurium braun lipoprotein (Lpp) and acetyltransferase (MsbB) mutants. Front. Cell. Infect. Microbiol. 6, 148.
Fields, P.I., Swanson, R.V., Haidaris, C.G., and Heffron, F. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. USA 83, 5189–5193.
Govoni, G. and Gros, P. 1998. Macrophage NRAMP1 and its role in resistance to microbial infections. Inflamm. Res. 47, 277–284.
Groszer, M., Erickson, R., Scripture-Adams, D.D., Lesche, R., Trumpp, A., Zack, J.A., Kornblum, H.I., Liu, X., and Wu, H. 2001. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science 294, 2186–2189.
Gut, A.M., Vasiljevic, T., Yeager, T., and Donkor, O.N. 2018. Salmonella infection — prevention and treatment by antibiotics and probiotic yeasts: A review. Microbiology 164, 1327–1344.
Haddadi, N., Lin, Y., Travis, G., Simpson, A.M., Nassif, N.T., and McGowan, E.M. 2018. PTEN/PTENP1: ‘Regulating the regulator of RTK-dependent PI3K/Akt signalling’, new targets for cancer therapy. Mol. Cancer 17, 37.
Hapfelmeier, S. and Hardt, W.D. 2005. A mouse model for S. typhimurium-induced enterocolitis. Trends Microbiol. 13, 497–503.
Howe, C., Kim, S.J., Mitchell, J., Im, E., Kim, Y.S., Kim, Y.S., and Rhee, S.H. 2018. Differential expression of tumor-associated genes and altered gut microbiome with decreased Akkermansia muciniphila confer a tumor-preventive microenvironment in intestinal epithelial pten-deficient mice. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 3746–3758.
Hsu, F. and Mao, Y. 2015. The structure of phosphoinositide phosphatases: Insights into substrate specificity and catalysis. Biochim. Biophys. Acta 1851, 698–710.
Im, E., Jung, J., Pothoulakis, C., and Rhee, S.H. 2014. Disruption of Pten speeds onset and increases severity of spontaneous colitis in IL10 -/- mice. Gastroenterology 147, 667–679.e610.
Jamaspishvili, T., Berman, D.M., Ross, A.E., Scher, H.I., De Marzo, A.M., Squire, J.A., and Lotan, T.L. 2018. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol. 15, 222–234.
Kagan, J.C. and Medzhitov, R. 2006. Phosphoinositide-mediated adaptor recruitment controls toll-like receptor signaling. Cell 125, 943–955.
Kaiser, P., Diard, M., Stecher, B., and Hardt, W.D. 2012. The streptomycin mouse model for Salmonella diarrhea: Functional analysis of the microbiota, the pathogen’s virulence factors, and the host’s mucosal immune response. Immunol. Rev. 245, 56–83.
Langlois, M.J., Roy, S.A., Auclair, B.A., Jones, C., Boudreau, F., Carrier, J.C., Rivard, N., and Perreault, N. 2009. Epithelial phosphatase and tensin homolog regulates intestinal architecture and secretory cell commitment and acts as a modifier gene in neoplasia. FASEB J. 23, 1835–1844.
Liu, H., Chen, F., Wu, W., Cao, A.T., Xue, X., Yao, S., Evans-Marin, H.L., Li, Y.Q., and Cong, Y. 2016. TLR5 mediates CD172α+ intestinal lamina propria dendritic cell induction of Th17 cells. Sci. Rep. 6, 22040.
Madison, B.B., Dunbar, L., Qiao, X.T., Braunstein, K., Braunstein, E., and Gumucio, D.L. 2002. cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J. Biol. Chem. 277, 33275–33283.
Que, W.C., Qiu, H.Q., Cheng, Y., Liu, M.B., and Wu, C.Y. 2018. PTEN in kidney cancer: A review and meta-analysis. Clin. Chim. Acta 480, 92–98.
Rhee, S.H., Kim, H., Moyer, M.P., and Pothoulakis, C. 2006. Role of MyD88 in phosphatidylinositol 3-kinase activation by flagellin/toll-like receptor 5 engagement in colonic epithelial cells. J. Biol. Chem. 281, 18560–18568.
Rhee, S.H., Ma, E.L., Lee, Y., Tache, Y., Pothoulakis, C., and Im, E. 2015. Corticotropin releasing hormone and urocortin 3 stimulate vascular endothelial growth factor expression through the cAMP/CREB pathway. J. Biol. Chem. 290, 26194–26203.
Stecher, B., Paesold, G., Barthel, M., Kremer, M., Jantsch, J., Stallmach, T., Heikenwalder, M., and Hardt, W.D. 2006. Chronic Salmonella enterica serovar Typhimurium-induced colitis and cholangitis in streptomycin-pretreated Nramp1 +/+ mice. Infect. Immun. 74, 5047–5057.
Tsolis, R.M., Kingsley, R.A., Townsend, S.M., Ficht, T.A., Adams, L.G., and Baumler, A.J. 1999. Of mice, calves, and men. Comparison of the mouse typhoid model with other Salmonella infections. Adv. Exp. Med. Biol. 473, 261–274.
Vecchio, L., Seke Etet, P.F., Kipanyula, M.J., Krampera, M., and Nwabo Kamdje, A.H. 2013. Importance of epigenetic changes in cancer etiology, pathogenesis, clinical profiling, and treatment: What can be learned from hematologic malignancies? Biochim. Biophys. Acta 1836, 90–104.
Vijayan, A., Rumbo, M., Carnoy, C., and Sirard, J.C. 2018. Compartmentalized antimicrobial defenses in response to flagellin. Trends Microbiol. 26, 423–435.
Yu, M., Trobridge, P., Wang, Y., Kanngurn, S., Morris, S.M., Knoblaugh, S., and Grady, W.M. 2014. Inactivation of TGF-β signaling and loss of PTEN cooperate to induce colon cancer in vivo. Oncogene 33, 1538–1547.
Acknowledgements
This research was supported by grants from Oakland University and the National Institutes of Health (DK079015, S.H.R) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1A2C1010536 to E.I.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest with the contents of this article.
Rights and permissions
About this article
Cite this article
Howe, C., Mitchell, J., Kim, S.J. et al. Pten gene deletion in intestinal epithelial cells enhances susceptibility to Salmonella Typhimurium infection in mice. J Microbiol. 57, 1012–1018 (2019). https://doi.org/10.1007/s12275-019-9320-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-019-9320-3