Abstract
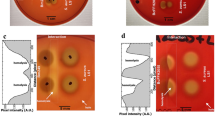
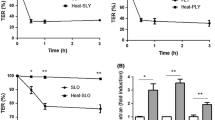
One of the advantages for initial survival of inhaled fungal spores in the respiratory tract is the ability for iron acquisition via hemolytic factor-production. To examine the ability of indoor Aspergillus and Penicillium affecting hemolysis, the secreted factors during the growth of thirteen strains from eight species were characterized in vitro for their hemolytic activity (HA) and CAMP-like reaction. The hemolytic index of HA on human blood agar of Aspergillus micronesiensis, Aspergillus wentii, Aspergillus westerdijkiae, Penicillium citrinum, Penicillium copticola, Penicillium paxilli, Penicillium steckii, and Penicillium sumatrense were 1.72 ± 0.34, 1.61 ± 0.41, 1.69 ± 0.16, 1.58 ± 0.46, 3.10 ± 0.51, 1.22 ± 0.19, 2.55 ± 0.22, and 1.90 ± 0.14, respectively. The secreted factors of an Aspergillus wentii showed high HA when grown in undernourished broth at 25°C at an exponential phase and were heat sensitive. Its secreted proteins have an estimated relative molecular weight over 50 kDa. Whereas, the factors of Penicillium steckii were secreted in a similar condition at a late exponential phase but showed low HA and heat tolerance. In a CAMP-like test with sheep blood, the synergistic hemolytic reactions between most tested mold strains and Staphylococcus aureus were identified. Moreover, the enhancement of α-hemolysis of Staphylococcus aureus could occur through the interaction of Staphylococcus aureus-sphingomyelinase and CAMP-like factors secreted from Aspergillus micronesiensis. Further studies on the characterization of purified hemolytic- and CAMP-like-factors secreted from Aspergillus wentii and Aspergillus micronesiensis may lead to more understanding of their involvement of hemolysis and cytolysis for fungal survival prior to pathogenesis.
Similar content being viewed by others
References
Atagazli, L., Greenhill, A.R., Melrose, W., Pue, A.G., and Warner, J.M. 2010. Is Penicillium citrinum implicated in sago hemolytic disease? SE. J. Trop. Med.41, 641–646.
Barratt, R.W., Johnson, G.B., and Ogata, W.N. 1965. Wild-type and mutant stocks of Aspergillus nidulans. Genetics52, 233–246.
Bernheimer, A.W., Linder, R., and Avigad, L.S. 1979. Nature and mechanism of action of the CAMP protein of group B streptococci. Infect. Immun.23, 838–844.
Christie, R., Atkins, N.E., and Munch-Petersen, E. 1944. A note on a lytic phenomenon shown by group B streptococci. Aust. J. Exp. Biol. Med. Sci.22, 197–200.
Deriu, E., Liu, J.Z., Pezeshki, M., Edwards, R.A., Ochoa, R.J., Contreras, H., Libby, S.J., Fang, F.C., and Raffatellu, M. 2013. Probiotic bacteria reduce Salmonella Typhimurium intestinal colonization by competing for iron. Cell Host Microbe14, 26–37.
Doegen, A., Guemral, R., and Ilkit, M. 2015. Haemolytic and cohaemolytic (CAMP-like) activity in Dermatophytes. Mycoses58, 40–47.
Donohue, M., Chung, Y., Magnuson, M.L., Ward, M., Selgrade, M.J., and Vesper, S. 2005. Hemolysin chrysolysin from Penicillium chrysogenum promotes inflammatory response. Int. J. Hyg. Environ. Health208, 279–285.
Donohue, M., Wei, W., Wu, J., Zawia, N.H., Hud, N., De Jesus, V., Schmechel, D., Hettick, J.M., Beezhold, D.H., and Vesper, S. 2006. Characterization of nigerlysin, hemolysin produced by Aspergillus niger, and effect on mouse neuronal cells in vitro. Toxicology219, 150–155.
Eduard, W. 2009. Fungal spores: a critical review of the toxicological and epidemiological evidence as a basis for occupational exposure limit setting. Crit. Rev. Toxicol.39, 799–864.
Houbraken, J.A.M.P., Frisvad, J.C., and Samson, R.A. 2010. Taxonomy of Penicillium citrinum and related species. Fungal Divers.44, 117–133.
Juntachai, W., Kummasook, A., Mekaprateep, M., and Kajiwara, S. 2014. Identification of the haemolytic activity of Malassezia species. Mycoses57, 163–168.
Lacey, M.E. and West, J.S. 2006. The Aerobiology Pathway, pp. 15–34. In Lacey, M.E. and West, J.S. (eds.), The Air Spora: A manual for catching and identifying airborne biological particles. Springer, New York, USA.
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Lo, C.W., Lai, Y.K., Liu, Y.T., Gallo, R.L., and Huang C.M. 2011. Staphylococcus aureus hijacks a skin commensal to intensify its virulence: immunization targeting β-hemolysin and CAMP factor. J. Invest. Dermatol.131, 401–409.
Luo, G., Samaranayake, L.P., and Yau, J.Y. 2001. Candida species exhibit differential in vitro hemolytic activities. J. Clin. Microbiol.39, 2971–2974.
Malcok, H.K., Aktas, E., Ayyildiz, A., Yigit, N., and Yazgi, H. 2009. Hemolytic activities of the Candida species in liquid medium. Eurasian J. Med.41, 95–98.
Malmstrom, J., Christophersen, C., and Frisvad, J.C. 2000. Secondary metabolites characteristic of Penicillium citrinum, Penicillium steckii and related species. Phytochemistry54, 301–309.
Nayak, A.P., Green, B.J., and Beezhold, D.H. 2013. Fungal hemolysins. Med. Mycol.51, 1–16.
Nguyen, L.D., Viscogliosi, E., and Delhaes, L. 2015. The lung mycobiome: an emerging field of the human respiratory microbiome. Front. Microbiol.6, 89.
Ramsey, M.M., Freire, M.O., Gabrilska, R.A., Rumbaugh, K.P., and Lemon, K.P. 2016. Staphylococcus aureus shifts toward commensalism in response to Corynebacterium species. Front. Microbiol.7, 1230.
Schaufuss, P., Brasch, J., and Steller, U. 2005. Dermatophytes can trigger cooperative (CAMP-like) haemolytic reactions. Br. J. Dermatol.153, 584–590.
Schaufuss, P., Müller, F., and Valentin-Weigand, P. 2007. Isolation and characterization of a haemolysin from Trichophyton mentagrophytes. Vet. Microbiol.122, 342–349.
Schaufuss, P. and Steller, U. 2003. Hemolytic activities of Trichophyton species. Med. Mycol.41, 511–516.
Shipton, W.A., Greenhill, A.R., and Warner, J.M. 2013. Sago hemolytic diseases: towards understanding a novel food-borne toxicosis. PNG. Med. J.56, 166–177.
Van Emon, J.M., Reed, A.W., Yike, I., and Vesper, S.J. 2003. ELISA measurement of stachylysin in serum to quantify human exposures to the indoor mold Stachybotrys chartarum. J. Occup. En viron. Med.45, 582–591.
Vanittanakom, N., Vanittanakom, P., and Hay, R.J. 2002. Rapid identification of Penicillium marneffei by PCR-based detection of specific sequences on the rRNA gene. J. Clin. Microbiol.40, 1739–1742.
Vesper, S.J. and Vesper, M.J. 2002. Stachylysin may be a cause of hemorrhaging in humans exposed to Stachybotrys chartarum. Infect. Immun.70, 2065–2069.
Wartenberg, D., Lapp, K., Jacobsen, I.D., Dahse, H.M., Kniemever, O., Heinekamp, T., and Krakhage, A.A. 2011. Secretome analysis of Aspergillus fumigatus reveals Asp-hemolysin as a major secreted protein. Int. J. Med. Microbiol.301, 602–611.
White, T.J., Bruns, T., Lee, S., and Taylor, J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, pp. 315–322. In Innis, M.A., Gelfand, D.H., Sninsky, J.J., and White, T.J. (eds.), PCR Protocols. Academic Press, San Diego, USA.
Acknowledgements
This research was funded by the Faculty of Medicine, Chiang Mai University. We would like to thank Assoc. Prof. Prasit Tharavichitkul and Miss Piyawan Takarn for providing bacterial samples, Assist. Prof. Bongkotwon Sutabhaha for providing a modified air sampler and mold identification method, and Miss Siriporn Jongkae for her help in air sampling and mold identification.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kaveemongkonrat, S., Duangsonk, K., Houbraken, J. et al. Partial characteristics of hemolytic factors secreted from airborne Aspergillus and Penicillium, and an enhancement of hemolysis by Aspergillus micronesiensis CAMP-like factor via Staphylococcus aureus-sphingomyelinase. J Microbiol. 57, 1086–1094 (2019). https://doi.org/10.1007/s12275-019-9133-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-019-9133-4