Abstract
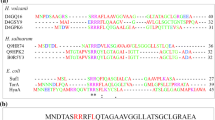
Signal peptide (SP) plays a pivotal role in protein translocation. Lipoprotein- and twin arginine translocase (Tat) dependent signal peptides were studied in All3087, a homolog of competence protein of Synechocystis PCC6803 and in two putative alkaline phosphatases (ALPs, Alr2234 and Alr4976), respectively. In silico analysis of All3087 is shown to possess the characteristics feature of competence proteins such as helix-hairpin-helix, N and C-terminal HKD endonuclease domain, calcium binding domain and N-terminal lipoprotein signal peptide. The SP recognition-cleavage site in All3087 was predicted (AIA-AC) using SignalP while further in-depth analysis using Pred-Lipo and WebLogo analysis for consensus sequence showed it as IAA-C. Activities of putative ALPs were confirmed by heterologous overexpression, activity assessment and zymogram analysis. ALP activity in Anabaena remains cell bound in log-phase, but during late log/stationary phase, an enhanced ALP activity was detected in extracellular milieu. The enhancement of ALP activity during stationary phase was not only due to inorganic phosphate limitation but also contributed by the presence of novel bipartite Tat-SP. The Tat signal transported the folded active ALPs to the membrane, followed by anchoring into the membrane and successive cleavage enabling transportation of the ALPs to the extracellular milieu, because of bipartite architecture and processing of transit Tat-SP.
Similar content being viewed by others
References
Barbrook, A.C., Packer, J.C., and Howe, C.J. 1993. Components of the protein translocation machinery in the thermophilic cyanobacterium Phormidium laminosum. Biochem. Biophys. Res. Commun. 197, 874–877.
Blobel, G. and Dobberstein, B. 1975. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membranebound ribosomes of murine myeloma. J. Cell Biol. 67, 835–851.
Bolhuis, A. 2002. Protein transport in the halophilic archaeon Halobacterium sp. Nrc-1: A major role for the twin-arginine translocation pathway? Microbiology 148, 3335–3346.
Castenholz, R. 1988. Culturing methods for cyanobacteria. Method Enzymol. 167, 68–93.
Chaurasia, A.K., Adhya, T.K., and Apte, S.K. 2013. Engineering bacteria for bioremediation of persistent organochlorine pesticide lindane (gamma-hexachlorocyclohexane). Bioresour. Technol. 149, 439–445.
Chaurasia, A.K. and Apte, S.K. 2011. Improved eco-friendly recombinant Anabaena sp. strain PCC7120 with enhanced nitrogen biofertilizer potential. Appl. Environ. Microbiol. 77, 395–399.
Chen, X., Van Valkenburgh, C., Fang, H., and Green, N. 1999. Signal peptides having standard and nonstandard cleavage sites can be processed by imp1p of the mitochondrial inner membrane protease. J. Biol. Chem. 274, 37750–37754.
Cohen, S.N., Chang, A.C., and Hsu, L. 1972. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc. Natl. Acad. Sci. USA 69, 2110–2114.
Dalbey, R.E., Chen, M., Jiang, F., and Samuelson, J.C. 2000. Understanding the insertion of transporters and other membrane proteins. Curr. Opin. Cell Biol. 12, 435–442.
Dalbey, R.E. and Kuhn, A. 2000. Evolutionarily related insertion pathways of bacterial, mitochondrial, and thylakoid membrane proteins. Annu. Rev. Cell Dev. Biol. 16, 51–87.
Emanuelsson, O., Brunak, S., von Heijne, G., and Nielsen, H. 2007. Locating proteins in the cell using Targetp, SignalP and related tools. Nat. Protoc. 2, 953–971.
Fiske, C.H. and Subbarao, Y. 1925. The colorimetric determination of phosphorus. J. Biol. Chem. 66, 375–400.
Gruhler, A., Arnold, I., Seytter, T., Guiard, B., Schwarz, E., Neupert, W., and Stuart, R.A. 1997. N-terminal hydrophobic sorting signals of preproteins confer mitochondrial hsp70 independence for import into mitochondria. J. Biol. Chem. 272, 17410–17415.
Hall, T.A. 1999. Bioedit: A user-friendly biological sequence alignment editor and analysis program for window 95/98/nt. Nucleic Acids Symp. Ser. 41, 95–98.
Hartl, F.U., Lecker, S., Schiebel, E., Hendrick, J.P., and Wickner, W. 1990. The binding cascade of secB to seca to secY/E mediates preprotein targeting to the E. coli plasma membrane. Cell 63, 269–279.
Hitchcock, A., Hall, S.J., Myers, J.D., Mulholland, F., Jones, M.A., and Kelly, D.J. 2010. Roles of the twin-arginine translocase and associated chaperones in the biogenesis of the electron transport chains of the human pathogen Campylobacter jejuni. Microbiology 156, 2994–3010.
Joshi, M.V., Mann, S.G., Antelmann, H., Widdick, D.A., Fyans, J.K., Chandra, G., Hutchings, M.I., Toth, I., Hecker, M., Loria, R., et al. 2010. The twin arginine protein transport pathway exports multiple virulence proteins in the plant pathogen Streptomyces scabies. Mol. Microbiol. 77, 252–271.
Kaneko, T., Nakamura, Y., Wolk, C.P., Kuritz, T., Sasamoto, S., Watanabe, A., Iriguchi, M., Ishikawa, A., Kawashima, K., Kimura, T., et al. 2001. Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 8, 205–213; 227–253.
Kelley, L.A. and Sternberg, M.J. 2009. Protein structure prediction on the web: A case study using the phyre server. Nat. Protoc. 4, 363–371.
Kushnareva, Y.E., Polster, B.M., Sokolove, P.M., Kinnally, K.W., and Fiskum, G. 2001. Mitochondrial precursor signal peptide induces a unique permeability transition and release of cytochrome c from liver and brain mitochondria. Arch. Biochem. Biophys. 386, 251–260.
Lam, S.L., Kirby, S., and Schryvers, A.B. 2003. Foreign signal peptides can constitute a barrier to functional expression of periplasmic proteins in Haemophilus influenzae. Microbiology 149, 3155–3164.
Ling Lin, F., Zi Rong, X., Wei Fen, L., Jiang Bing, S., Ping, L., and Chun Xia, H. 2007. Protein secretion pathways in Bacillus subtilis: implication for optimization of heterologous protein secretion. Biotechnol. Adv. 25, 1–12.
Mackinney, G. 1941. Absorption of light by chlorophyll solutions. J. Biol. Chem. 140, 315–322.
Mandel, M. and Higa, A. 1970. Calcium-dependent bacteriophage DNA infection. J. Mol. Biol. 53, 159–162.
Mariscal, V., Herrero, A., and Flores, E. 2007. Continuous periplasm in a filamentous, heterocyst-forming cyanobacterium. Mol. Microbiol. 65, 1139–1145.
Nakai, M., Nohara, T., Sugita, D., and Endo, T. 1994. Identification and characterization of the sec-a protein homologue in the cyanobacterium Synechococcus PCC7942. Biochem. Biophys. Res. Commun. 200, 844–851.
Nilgiriwala, K.S., Alahari, A., Rao, A.S., and Apte, S.K. 2008. Cloning and overexpression of alkaline phosphatase phok from Sphingomonas sp. strain BSAR-1 for bioprecipitation of uranium from alkaline solutions. Appl. Environ. Microbiol. 74, 5516–5523.
Palmer, T. and Berks, B.C. 2012. The twin-arginine translocation (Tat) protein export pathway. Nat. Rev. Microbiol. 10, 483–496.
Pettersen, E.F., Goddard, T.D., Huang, C.C., Couch, G.S., Greenblatt, D.M., Meng, E.C., and Ferrin, T.E. 2004. UCSF chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612.
Pugsley, A.P., d’ Enfert, C., Reyss, I., and Kornacker, M.G. 1990. Genetics of extracellular protein secretion by Gram-negative bacteria. Annu. Rev. Genet. 24, 67–90.
Raghavan, P.S., Rajaram, H., and Apte, S.K. 2013. N-terminal processing of membrane-targeted mnsod and formation of multiple active superoxide dismutase dimers in the nitrogen-fixing cyanobacterium Anabaena sp. strain PCC7120. FEBS J. 280, 4827–4838.
Rajaram, H., Chaurasia, A.K., and Apte, S.K. 2014. Cyanobacterial heat-shock response: Role and regulation of molecular chaperones. Microbiology 160, 647–658.
Ravn, P., Arnau, J., Madsen, S.M., Vrang, A., and Israelsen, H. 2003. Optimization of signal peptide sp310 for heterologous protein production in Lactococcus lactis. Microbiology 149, 2193–2201.
Rezwan, M., Grau, T., Tschumi, A., and Sander, P. 2007. Lipoprotein synthesis in mycobacteria. Microbiology 153, 652–658.
Shackleton, J.B. and Robinson, C. 1991. Transport of proteins into chloroplasts. The thylakoidal processing peptidase is a signaltype peptidase with stringent substrate requirements at the -3 and -1 positions. J. Biol. Chem. 266, 12152–12156.
Simonen, M. and Palva, I. 1993. Protein secretion in Bacillus species. Microbiol. Rev. 57, 109–137.
Spence, E., Sarcina, M., Ray, N., Moller, S.G., Mullineaux, C.W., and Robinson, C. 2003. Membrane-specific targeting of green fluorescent protein by the tat pathway in the cyanobacterium Synechocystis PCC6803. Mol. Microbiol. 48, 1481–1489.
Stanley, N.R., Palmer, T., and Berks, B.C. 2000. The twin arginine consensus motif of tat signal peptides is involved in sec-independent protein targeting in Escherichia coli. J. Biol. Chem. 275, 11591–11596.
Sutcliffe, I.C. and Harrington, D.J. 2002. Pattern searches for identification of putative lipoprotein genes in Gram-positive bacterial genomes. Microbiology 148, 2065–2077.
Tjalsma, H., Antelmann, H., Jongbloed, J.D., Braun, P.G., Darmon, E., Dorenbos, R., Dubois, J.Y., Westers, H., Zanen, G., Quax, W.J., et al. 2004. Proteomics of protein secretion by Bacillus subtilis: Separating the “secrets” of the secretome. Microbiol. Mol. Biol. Rev. 68, 207–233.
Tjalsma, H., Bolhuis, A., Jongbloed, J.D., Bron, S., and van Dijl, J.M. 2000. Signal peptide-dependent protein transport in Bacillus subtilis: A genome-based survey of the secretome. Microbiol. Mol. Biol. Rev. 64, 515–547.
Tjalsma, H., Zanen, G., Venema, G., Bron, S., and van Dijl, J.M. 1999. The potential active site of the lipoprotein-specific (type ii) signal peptidase of Bacillus subtilis. J. Biol. Chem. 274, 28191–28197.
Ungermann, C., Neupert, W., and Cyr, D.M. 1994. The role of Hsp70 in conferring unidirectionality on protein translocation into mitochondria. Science 266, 1250–1253.
von Heijne, G. 1990. The signal peptide. J. Membr. Biol. 115, 195–201.
Wexler, M., Bogsch, E.G., Klosgen, R.B., Palmer, T., Robinson, C., and Berks, B.C. 1998. Targeting signals for a bacterial sec-independent export system direct plant thylakoid import by the delta pH pathway. FEBS Lett. 431, 339–342.
Wickner, W. and Schekman, R. 2005. Protein translocation across biological membranes. Science 310, 1452–1456.
Widdick, D.A., Dilks, K., Chandra, G., Bottrill, A., Naldrett, M., Pohlschröder, M., and Palmer, T. 2006. The twin-arginine translocation pathway is a major route of protein export in Streptomyces coelicolor. Proc. Natl. Acad. Sci. USA 103, 17927–17932.
Yoshihara, S., Geng, X., Okamoto, S., Yura, K., Murata, T., Go, M., Ohmori, M., and Ikeuchi, M. 2001. Mutational analysis of genes involved in pilus structure, motility and transformation competency in the unicellular motile cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 42, 63–73.
Zanen, G., Houben, E.N., Meima, R., Tjalsma, H., Jongbloed, J.D., Westers, H., Oudega, B., Luirink, J., van Dijl, J.M., and Quax, W.J. 2005. Signal peptide hydrophobicity is critical for early stages in protein export by Bacillus subtilis. FEBS J. 272, 4617–4630.
Zhang, X.P. and Glaser, E. 2002. Interaction of plant mitochondrial and chloroplast signal peptides with the Hsp70 molecular chaperone. Trends Plant Sci. 7, 14–21.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplemental material for this article may be found at http://www.springerlink.com/content/120956.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kumari, S., Chaurasia, A.K. In silico analysis and experimental validation of lipoprotein and novel Tat signal peptides processing in Anabaena sp. PCC7120. J Microbiol. 53, 837–846 (2015). https://doi.org/10.1007/s12275-015-5281-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-015-5281-3