Abstract
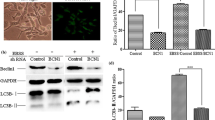
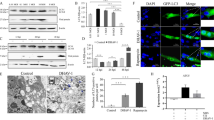
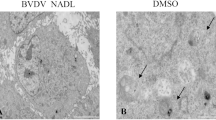
Bovine viral diarrhea virus (BVDV) is an enveloped, positive-sense, single-stranded RNA virus that belongs to the genus Pestivirus (Flaviviridae). The signaling pathways and levels of signaling molecules are altered in Madin-Darby Bovine Kidney (MDBK) cells infected with BVDV. Autophagy is a conservative biological degradation pathway that mainly eliminates and degrades damaged or superfluous organelles and macromolecular complexes for intracellular recycling in eukaryotic cells. Autophagy can also be induced as an effective response to maintain cellular homeostasis in response to different stresses, such as nutrient or growth factor deprivation, hypoxia, reactive oxygen species exposure and pathogen infection. However, the effects of BVDV infection on autophagy inMDBK cells remain unclear. Therefore, we performed an analysis of autophagic activity after BVDV NADL infection using real-time PCR, electron microscopy, laser confocal microscopy, and Western blotting analysis. The results demonstrated that BVDV NADL infection increased autophagic activity and significantly elevated the expression levels of the autophagy-related genes Beclin1 and ATG14 inMDBK cells. However, the knockdown of Beclin1 and ATG14 by RNA interference (RNAi) did not affect BVDV NADL infection-related autophagic activity. These findings provided a novel perspective to elaborate the effects of viral infection on the host cells.
Similar content being viewed by others
Refrerences
Ashford, T.P. and Porter, K.R. 1962. Cytoplasmic components in hepatic cell lysosomes. J. Cell Biol. 12, 198–202.
Carpenter, J.E., Jackson, W., Benetti, L., and Grose, C. 2011. Autophagosome formation during varicella-zoster virus infection following endoplasmic reticulum stress and the unfolded protein response. J. Virol. 85, 9414–9424.
Deretic, V. 2006. Autophagy as an immune defense mechanism. Curr. Opin. Immunol. 18, 375–382.
Dreux, M. and Chisari, F.V. 2010. Viruses and the autophagy machinery. Cell Cycle 9, 1295–1307.
Dreux, M., Gastaminza, P., Wieland, S.F., and Chisari, F.V. 2009. The autophagy machinery is required to initiate hepatitis C virus replication. Proc. Natl. Acad. Sci. USA 106, 14046–14051.
Ferdous, A., Battiprolu, P.K., Ni, Y.G., Rothermel, B.A., and Hill, J.A. 2010. FoxO, autophagy, and cardiac remodeling. J. Cardiovasc. Transl. Res. 3, 355–364.
Gangodkar, S., Jain, P., Dixit, N., Ghosh, K., and Basu, A. 2010. Dengue virus-induced autophagosomes and changes in endomembrane ultrastructure imaged by electron tomography and whole-mount grid-cell culture techniques. J. Electron Microsc. 59, 503–511.
Guo, F., Zhang, H., Chen, C., Hu, S., Wang, Y., Qiao, J., Ren, Y., Zhang, K., Wang, Y., and Du, G. 2012. Autophagy favors Brucella melitensis survival in infected macrophages. Cell Mol. Biol. Lett. 17, 249–257.
He, C. and Klionsky, D.J. 2009. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 43, 67–93.
Heaton, N.S. and Randall, G. 2010. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe 8, 422–432.
Heaton, N.S. and Randall, G. 2011. Dengue virus and autophagy. Viruses 3, 1332–1341.
Huang, H., Kang, R., Wang, J., Luo, G., Yang, W., and Zhao, Z. 2012. Hepatitis C virus inhibits AKT-tuberous sclerosis complex (TSC), the mechanistic target of rapamycin (mTOR) pathway, through endoplasmic reticulum stress to induce autophagy. Autophagy 9, 175–195.
Hulst, M.M., van Gennip, H.G., and Moormann, R.J. 2000. Passage of classical swine fever virus in cultured swine kidney cells selects virus variants that bind to heparan sulfate due to a single amino acid change in envelope protein Erns. J. Virol. 74, 9553–9561.
Kadowaki, M. and Karim, M.R. 2009. Cytosolic LC3 ratio as a quantitative index of macroautophagy. Methods Enzymol. 452, 199–213.
Kang, R., Zeh, H.J., Lotze, M.T., and Tang, D. 2011. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 18, 571–580.
Ke, P.Y. and Chen, S.S.L. 2011a. Autophagy: a novel guardian of HCV against innate immune response. Autophagy 7, 533–535.
Ke, P.Y. and Chen, S.S.L. 2011b. Activation of the unfolded protein response and autophagy after hepatitis C virus infection suppresses innate antiviral immunity in vitro. J. Clin. Invest. 121, 37.
Klionsky, D.J. 2007. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell Biol. 8, 931–937.
Klionsky, D.J., Abdalla, F.C., Abeliovich, H., Abraham, R.T., Acevedo-Arozena, A., Adeli, K., Agholme, L., Agnello, M., Agostinis, P., and Aguirre-Ghiso, J.A. 2012. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8, 445–544.
Lee, Y.R., Lei, H.Y., Liu, M.T., Wang, J.R., Chen, S.H., Jiang-Shieh, Y.F., Lin, Y.S., Yeh, T.M., Liu, C.C., and Liu, H.S. 2008. Autophagic machinery activated by dengue virus enhances virus replication. Virology 374, 240–248.
Li, M., Jiang, X., Liu, D., Na, Y., Gao, G.F., and Xi, Z. 2008. Autophagy protects lncap cells under androgen deprivation conditions. Autophagy 4, 54–60.
Li, J., Liu, Y., Wang, Z., Liu, K., Wang, Y., Liu, J., Ding, H., and Yuan, Z. 2011. Subversion of cellular autophagy machinery by hepatitis B virus for viral envelopment. J. Virol. 85, 6319–6333.
Livak, K.J. and Schmittgen, T.D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25, 402–408.
Mateo, R., Nagamine, C.M., Spagnolo, J., Méndez, E., Rahe, M., Gale, M., Yuan, J., and Kirkegaard, K. 2013. Inhibition of cellular autophagy deranges dengue virion maturation. J. Virol. 87, 1312–1321.
Mehrpour, M., Esclatine, A., Beau, I., and Codogno, P. 2010. Overview of macroautophagy regulation in mammalian cells. Cell Res. 20, 748–762.
Mendez, E., Ruggli, N., Collett, M.S., and Rice, C.M. 1998. Infectious bovine viral diarrhea virus (strain NADL) RNA from stable cDNA clones: a cellular insert determines NS3 production and viral cytopathogenicity. J. Virol. 72, 4737–4745.
Meyers, G., Stoll, D., and Gunn, M. 1998. Insertion of a sequence encoding light chain 3 of microtubule-associated proteins 1A and 1B in a pestivirus genome: connection with virus cytopathogenicity and induction of lethal disease in cattle. J. Virol. 72, 4139–4148.
Mizui, T., Yamashina, S., Tanida, I., Takei, Y., Ueno, T., Sakamoto, N., Ikejima, K., Kitamura, T., Enomoto, N., and Sakai, T. 2010. Inhibition of hepatitis C virus replication by chloroquine targeting virus-associated autophagy. J. Gastroenterol. 45, 195–203.
Mizushima, N. 2007. Autophagy: process and function. Genes Dev. 21, 2861–2873.
Mizushima, N., Levine, B., Cuervo, A.M., and Klionsky, D.J. 2008. Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075.
Obara, K. and Ohsumi, Y. 2011. Atg14: a key player in orchestrating autophagy. Int. J. Cell Biol. 2011, 71345.
Orvedahl, A., MacPherson, S., Sumpter Jr., R., Talloczy, Z., Zou, Z., and Levine, B. 2010. Autophagy protects against Sindbis virus infection of the central nervous system. Cell Host Microbe 7, 115–127.
Perdrizet, J.A., Rebhun, W.C., Dubovi, E.J., and Donis, R.O. 1987. Bovine virus diarrhea-clinical syndromes in dairy herds. Cornell. Vet. 77, 46–74.
Reed, L.J. and Muench, H. 1938. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 27, 493–497.
Ridpath, J.F., Bolin, S.R., and Dubovi, E.J. 1994. Segregation of bovine viral diarrhea virus into genotypes. Virology 205, 66–74.
Rodriguez-Rocha, H., Gomez-Gutierrez, J.G., Garcia-Garcia, A., Rao, X.M., Chen, L., McMasters, K.M., and Zhou, H.S. 2011. Adenoviruses induce autophagy to promote virus replication and oncolysis. Virology 416, 9–15.
Sir, D., Kuo, C.-f., Tian, Y., Liu, H.M., Huang, E.J., Jung, J.U., Machida, K., and Ou, J.-h.J. 2012. Replication of hepatitis C virus RNA on autophagosomal membranes. J. Biol. Chem. 287, 18036–18043.
Tanida, I., Ueno, T., and Kominami, E. 2008. LC3 and autophagy. Methods Mol. Biol. 445, 77–88.
Wong, J., Zhang, J., Si, X., Gao, G., Mao, I., McManus, B.M., and Luo, H. 2008. Autophagosome supports coxsackievirus B3 replication in host cells. J. Virol. 82, 9143–9153.
Xi, X., Zhang, X., Wang, B., Wang, T., Wang, J., Huang, H., Wang, J., Jin, Q., and Zhao, Z. 2013. The interplays between autophagy and apoptosis induced by enterovirus 71. PLoS ONE 8, e56966.
Zhang, Y., Li, Z., Ge, X., Guo, X., and Yang, H. 2011. Autophagy promotes the replication of encephalomyocarditis virus in host cells. Autophagy 7, 613–628.
Zhao, Z., Fux, B., Goodwin, M., Dunay, I.R., Strong, D., Miller, B.C., Cadwell, K., Delgado, M.A., Ponpuak, M., Green, K.G., and et al. 2008. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe 4, 458–469.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, Q., Shi, H., Ren, Y. et al. Bovine viral diarrhea virus infection induces autophagy in MDBK cells. J Microbiol. 52, 619–625 (2014). https://doi.org/10.1007/s12275-014-3479-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-014-3479-4