Abstract
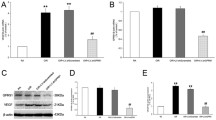
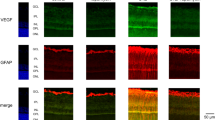
Treatment of human retinal microvascular endothelial cells (HRMECs) with vascular endothelial growth factor 165 (VEGF165) increased hypoxia-inducible factor 1α (HIF-1α), VEGF, and glucose transporter 1 (Glut-1) mRNA expression and Glut-1 protein localization to the membrane. In contrast, treatment of human retinal pigment epithelium cells with VEGF165 did not induce HIF-1α, VEGF, and Glut-1 gene expression. Microvascular endothelial cells are surrounded by astrocytic end feet in the retina. Astrocyte-derived A-kinase anchor protein 12 overexpression during hypoxia downregulated VEGF secretion, and this conditioned medium reduced VEGF and Glut-1 expression in HRMECs, suggesting that communications between astrocytes and endothelial cells may be the determinants of the blood vessel network. In HRMECs, HIF-1α small interfering RNA transfection blocked the VEGF165-mediated increase in VEGF and Glut-1 gene expression. Inhibition of protein kinase C (PKC) with inhibitor GF109203X or with a small interfering RNA targeting PKCζ attenuated the VEGF165-induced Glut-1 protein expression and VEGF and Glut-1 mRNA expression. In addition, results of an immunoprecipitation assay imply an interaction between VEGF receptor 2 (VEGFR2) and PKCζ in HRMECs. Therefore, VEGF secretion by hypoxic astrocytes may upregulate HIF-1α gene expression, inducing VEGF and Glut-1 expression via the VEGFR2–PKCζ axis in HRMECs.




Similar content being viewed by others
References
Bagli E, Stefaniotou M, Morbidelli L, Ziche M, Psillas K, Murphy C, Fotsis T (2004) Luteolin inhibits vascular endothelial growth factor-induced angiogenesis; inhibition of endothelial cell survival and proliferation by targeting phosphatidylinositol 3′-kinase activity. Cancer Res 64:7936–7946
Choi YK, Kim KW (2008a) AKAP12 in astrocytes induces barrier functions in human endothelial cells through protein kinase Czeta. FEBS J 275:2338–2353
Choi YK, Kim KW (2008b) Blood-neural barrier: its diversity and coordinated cell-to-cell communication. BMB Rep 41:345–352
Choi YK, Kim JH, Kim WJ, Lee HY, Park JA, Lee SW, Yoon DK, Kim HH, Chung H, Yu YS, Kim KW (2007) AKAP12 regulates human blood-retinal barrier formation by downregulation of hypoxia-inducible factor-1alpha. J Neurosci 27:4472–4481
De Gooyer TE, Stevenson KA, Humphries P, Simpson DA, Curtis TM, Gardiner TA, Stitt AW (2006) Rod photoreceptor loss in Rho-/- mice reduces retinal hypoxia and hypoxia-regulated gene expression. Investig Ophthalmol Vis Sci 47:5553–5560
Deudero JJ, Caramelo C, Castellanos MC, Neria F, Fernandez-Sanchez R, Calabia O, Penate S, Gonzalez-Pacheco FR (2008) Induction of hypoxia-inducible factor 1α gene expression by vascular endothelial growth factor. J Biol Chem 283:11435–11444
Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y (1997) A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA 94:4273–4278
Epstein AC, Gleadle JM, Mcneill LA, Hewitson KS, O’rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ (2001) C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107:43–54
Fraisl P, Mazzone M, Schmidt T, Carmeliet P (2009) Regulation of angiogenesis by oxygen and metabolism. Dev Cell 16:167–179
Ghosh G, Subramanian IV, Adhikari N, Zhang X, Joshi HP, Basi D, Chandrashekhar YS, Hall JL, Roy S, Zeng Y, Ramakrishnan S (2010) Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-alpha isoforms and promotes angiogenesis. J Clin Investig 120:4141–4154
Gilbert RE, Vranes D, Berka JL, Kelly DJ, Cox A, Wu LL, Stacker SA, Cooper ME (1998) Vascular endothelial growth factor and its receptors in control and diabetic rat eyes. Lab Investig 78:1017–1027
Hughes JM, Groot AJ, Van Der Groep P, Sersansie R, Vooijs M, Van Diest PJ, Van Noorden CJ, Schlingemann RO, Klaassen I (2010) Active HIF-1 in the normal human retina. J Histochem Cytochem 58:247–254
Jo DH, Bae J, Chae S, Kim JH, Han JH, Hwang D, Lee SW, Kim JH (2016) Quantitative proteomics reveals beta2 integrin-mediated cytoskeletal rearrangement in vascular endothelial growth factor (VEGF)-induced retinal vascular hyperpermeability. Mol Cell Proteom 15:1681–1691
Kaelin WG Jr, Ratcliffe PJ (2008) Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 30:393–402
Kumagai AK, Glasgow BJ, Pardridge WM (1994) GLUT1 glucose transporter expression in the diabetic and nondiabetic human eye. Investig Ophthalmol Vis Sci 35:2887–2894
Kumagai AK, Vinores SA, Pardridge WM (1996) Pathological upregulation of inner blood-retinal barrier Glut1 glucose transporter expression in diabetes mellitus. Brain Res 706:313–317
Kuribayashi K, Nakamura K, Tanaka M, Sato T, Kato J, Sasaki K, Takimoto R, Kogawa K, Terui T, Takayama T, Onuma T, Matsunaga T, Niitsu Y (2007) Essential role of protein kinase C zeta in transducing a motility signal induced by superoxide and a chemotactic peptide, fMLP. J Cell Biol 176:1049–1060
Lee MS, Moon EJ, Lee SW, Kim MS, Kim KW, Kim YJ (2001) Angiogenic activity of pyruvic acid in in vivo and in vitro angiogenesis models. Cancer Res 61:3290–3293
Li X, Hahn CN, Parsons M, Drew J, Vadas MA, Gamble JR (2004) Role of protein kinase Czeta in thrombin-induced endothelial permeability changes: inhibition by angiopoietin-1. Blood 104:1716–1724
Luo R, Zhang W, Zhao C, Zhang Y, Wu H, Jin J, Zhang W, Grenz A, Eltzschig HK, Tao L, Kellems RE, Xia Y (2015) Elevated endothelial hypoxia-inducible factor-1alpha contributes to glomerular injury and promotes hypertensive chronic kidney disease. Hypertension 66:75–84
Mantych GJ, Hageman GS, Devaskar SU (1993) Characterization of glucose transporter isoforms in the adult and developing human eye. Endocrinology 133:600–607
Maxwell PH, Dachs GU, Gleadle JM, Nicholls LG, Harris AL, Stratford IJ, Hankinson O, Pugh CW, Ratcliffe PJ (1997) Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci USA 94:8104–8109
Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A (1993) High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 72:835–846
Minshall RD, Vandenbroucke EE, Holinstat M, Place AT, Tiruppathi C, Vogel SM, Van Nieuw Amerongen GP, Mehta D, Malik AB (2010) Role of protein kinase Czeta in thrombin-induced RhoA activation and inter-endothelial gap formation of human dermal microvessel endothelial cell monolayers. Microvasc Res 80:240–249
Mintz-Hittner HA, Kennedy KA, Chuang AZ, Group B-RC (2011) Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 364:603–615
Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3:721–732
Semenza GL (2007) Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE 2007:cm8
Sone H, Deo BK, Kumagai AK (2000) Enhancement of glucose transport by vascular endothelial growth factor in retinal endothelial cells. Investig Ophthalmol Vis Sci 41:1876–1884
Spaide RF, Fisher YL (2006) Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina 26:275–278
Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan-Ling T, Keshet E (1995) Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci 15:4738–4747
Van Kolen K, Slegers H (2006) Atypical PKCzeta is involved in RhoA-dependent mitogenic signaling by the P2Y(12) receptor in C6 cells. FEBS J 273:1843–1854
Wang GL, Jiang BH, Rue EA, Semenza GL (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92:5510–5514
Wechsler-Reya RJ, Barres BA (1997) Retinal development: communication helps you see the light. Curr Biol 7:R433–R436
West H, Richardson WD, Fruttiger M (2005) Stabilization of the retinal vascular network by reciprocal feedback between blood vessels and astrocytes. Development 132:1855–1862
Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO (2003) Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 22:1–29
Zhang P, Fu C, Hu Y, Dong C, Song Y, Song E (2015) C6-ceramide nanoliposome suppresses tumor metastasis by eliciting PI3K and PKCzeta tumor-suppressive activities and regulating integrin affinity modulation. Sci Rep 5:9275
Acknowledgements
This paper was supported by Konkuk University in 2017. Dr. Irwin H. Gelman (Roswell Park Cancer Institute, Buffalo, NY, USA) kindly provided the full-length rat Akap12 cDNA, and Dr. Kyu-Won Kim (Seoul National University, Seoul, Republic of Korea) kindly gave us the subcloned vector (pcDNA3/AKAP12).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Choi, Y. A positive circuit of VEGF increases Glut-1 expression by increasing HIF-1α gene expression in human retinal endothelial cells. Arch. Pharm. Res. 40, 1433–1442 (2017). https://doi.org/10.1007/s12272-017-0971-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-017-0971-5