Abstract
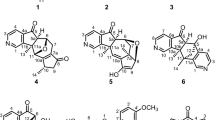
Three new minor pyrrole alkaloids, 3-[2-formyl-5-(hydroxymethyl)-1H-pyrrol-1-yl]pentanedioic acid (1), (2R)-[2-formyl-5-(hydroxymethyl)-1H-pyrrol-1-yl]-1-methoxy-1-oxobutanoic acid (2), and methyl (2R)-[2-formyl-5-(methoxymethyl)-1H-pyrrol-1-yl]-4-methylpentanoate (3) were isolated from the fruits of Lycium chinense Miller (Solanaceae), along with the known compound, methyl (2R)-[2-formyl-5-(methoxymethyl)-1H-pyrrol-1-yl]-3-(phenyl)propanoate (4). The structures of 1–4 were elucidated by analysis of their 1D- and 2D-NMR and HRMS data. The absolute configurations of 2–4, possessing a stereogenic center in each structure, were determined by comparison of their experimental electronic circular dichroism (ECD) with those of calculated ECD values.




Similar content being viewed by others
References
Amos RIJ, Gourlay BS, Molesworth PP, Smith JA, Sprod OR (2005) Annulation of pyrrole: application to the synthesis of indolizidine alkaloids. Tetrahedron 61:8226–8230
Barone V, Cossi MJ (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102:1995–2001
Bruhn T, Hemberger Y, Schaumlöffel A, Bringmann G (2010) SpecDis version 1.50. University of Wűerzburg, Wűerzburg
Chan TH, Lee SD (1983) 1,4-Dichloro-1,4-dimethoxybutane as a mild reagent for the conversion of primary amines to pyrroles. Synthesis of a pyrrole compound from tobacco. J Org Chem 48:3059–3061
Charles WJ, Qian T, Alexander ZJ (1991) Short, enantiogenic syntheses of (−)-indolizidine 167B and (+)-monomorine. J Am Chem Soc 113:3513–3518
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J Comput Chem 24:669–681
Dieter RK, Yu H (2000) A facile synthesis of polysubstituted pyrroles. Org Lett 2:2283–2286
Duke JA, Bogenschutz-Godwin MJ, duCellier J, Duke PAK (2002) Handbook of medicinal herbs. CRC Press, New York
Franc C, Denone F, Cuisiner C, Ghosez L (1999) A general synthesis of 2-formyl-3-arylpyrroles. Tetrahedron Lett 40:4555–4558
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE Jr., Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Revision D.01. Gaussian, Inc, Wallingford
Gaullier JM, Bazin M, Valla A, Giraud M, Santus RJ (1995) Amino acid-pyrrole N-conjugates; a new class of antioxidants II. Effectiveness of singlet oxygen quenching by luminescence measurements. J Photochem Photobiol B 30:195–200
Jefford CW, Sienkiewicz K, Thornton SR (1995) Short, enantiospecific syntheses of indolizidines 209B and 209D, and piclavine A from diethyl-L-glutamate. Helv Chim Acta 78:1511–1524
Josey AD, Jenner EL (1962) N-Functionally substituted pyrroles. J Org Chem 27:2466–2470
Kang YK (2001) Ab initio MO and density functional studies on trans and cis conformers of N-methylacetamide. J Mol Struct (Thoechem) 546:183–193
Kim SY, Choi YH, Huh H, Kim J, Kim YC, Lee HS (1997a) New antihepatotoxic cerebroside from Lycium chinense fruits. J Nat Prod 60:274–276
Kim SY, Kim HP, Huh H, Kim YC (1997b) Antihepatotoxic zeaxanthins from the fruits of Lycium chinense. Arch Pharmacal Res 20:529–532
Lin CC, Chuang SC, Lin JM, Yang JJ (1997) Evaluation of the antiinflammatory hepatoprotective and antioxidant activities of Lycium chinense from Taiwan. Phytomedicine 4:213–220
Liu JH, Yang QC, Mak TCW, Wong HNC (2000) Highly regioselective synthesis of 2,3,4-trisubstituted 1H-pyrroles: a formal total synthesis of lukianol A. J Org Chem 65:3587–3595
Liu WY, Zhang WD, Chen H, Gu ZB, Li TZ, Zhou YJ (2003) Pyrrole alkaloids from Bolbostemma paniculatum. J Asian Nat Prod Res 5:159–163
Park MJ, Kim SR, Huh H, Jung JH, Kim YC (1994) Betaine attenuates glutamate-induced neurotoxicity in primary cultured brain cells. Arch Pharmacal Res 17:343–347
Ragno R, Marshall GR, Di Santo R, Costi R, Massa S, Rompei R, Artico M (2000) Antimycobacterial pyrroles: synthesis, anti-Mycobacterium tuberculosis activity and QSAR studies. Bioorg Med Chem 8:1423–1432
Tressl R, Nittka C, Kersten E, Rewicki DJ (1995) Formation of isoleucine-specific Maillard products from [1-13C]-d-glucose and [1-13C]-D-fructose. J Agric Food Chem 43:1163–1169
Youn UJ, Kil YS, Nam JW, Lee YJ, Kim J, Lee D, Lee JH, Seo EK (2013) New pyrrole alkaloids with bulky N-alkyl side chains containing stereogenic centers from Lycium chinense. Helv Chim Acta 96:1482–1487
Acknowledgments
This paper (Youn UJ) was supported by [RP-Grant 2010 of Ewha Womans University].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Youn, U.J., Lee, J.Y., Kil, YS. et al. Identification of new pyrrole alkaloids from the fruits of Lycium chinense . Arch. Pharm. Res. 39, 321–327 (2016). https://doi.org/10.1007/s12272-015-0695-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-015-0695-3