Abstract
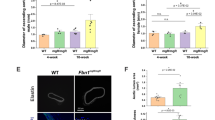
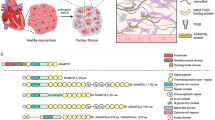
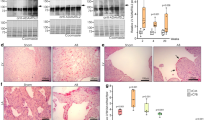
Fibrillin 1 (Fbn1) mutation causes Marfan syndrome (MFS) with thoracic aortic aneurysm (TAA) as the main complication. The mechanisms for extracellular matrix (ECM) homeostasis disruption in MFS TAA are unclear. Here, we found ECM-related gene secreted phosphoprotein 1 (Spp1) increased in Fbn1C1041G/+ mice using transcriptome sequencing and a distinct fibroblast subcluster with Spp1 as the strongest marker was identified with analysis of the MFS mouse aortic single-cell sequencing dataset. Immunostaining confirmed elevated Spp1 in adventitial fibroblasts, and Spp1 might regulate fibroblast and smooth muscle cell (SMC) communication primarily through Itga8/Itgb1. Then, we observed Spp1 reduced contractile genes Acta2 and Tagln expression in SMCs and increased collagen expression in fibroblasts, which might contribute to TAA development. Finally, we also found elevated SPP1 plasma level was associated with an increased risk of TAA in patients. Therefore, SPP1 may serve as a biomarker and therapeutic target for TAA.
Graphical abstract







Similar content being viewed by others
Abbreviations
- Fbn1:
-
Fibrillin-1
- TAA:
-
Thoracic aortic aneurysm
- ECM:
-
Extracellular matrix
- Spp1:
-
Secreted phosphoprotein 1
- SMC:
-
Smooth muscle cell
- MFS:
-
Marfan syndrome
- Itga8/Itgb1:
-
Integrin alpha 8/integrin beta 1
References
Hernándiz, A., Zúñiga, A., Valera, F., Domingo, D., Ontoria-Oviedo, I., Marí, J. F., Román, J. A., Calvo, I., Insa, B., Gómez, R., et al. (2021). Genotype FBN1/phenotype relationship in a cohort of patients with Marfan syndrome. Clinical genetics, 99, 269–280. https://doi.org/10.1111/cge.13879
Vanem, T. T., Geiran, O. R., Krohg-Sørensen, K., Røe, C., Paus, B., & Rand-Hendriksen, S. (2018). Survival, causes of death, and cardiovascular events in patients with Marfan syndrome. Molecular genetics & genomic medicine, 6, 1114–1123. https://doi.org/10.1002/mgg3.489
Cañadas, V., Vilacosta, I., Bruna, I., & Fuster, V. (2010). Marfan syndrome. Part 2: Treatment and management of patients. Nature reviews Cardiology, 7, 266–276. https://doi.org/10.1038/nrcardio.2010.31
Neptune, E. R., Frischmeyer, P. A., Arking, D. E., Myers, L., Bunton, T. E., Gayraud, B., Ramirez, F., Sakai, L. Y., & Dietz, H. C. (2003). Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nature genetics, 33, 407–411. https://doi.org/10.1038/ng1116
Ramirez, F., & Rifkin, D. B. (2003). Cell signaling events: A view from the matrix. Matrix biology : Journal of the International Society for Matrix Biology, 22, 101–107. https://doi.org/10.1016/s0945-053x(03)00002-7
Ramachandra, C. J., Mehta, A., Guo, K. W., Wong, P., Tan, J. L., & Shim, W. (2015). Molecular pathogenesis of Marfan syndrome. International journal of cardiology, 187, 585–591. https://doi.org/10.1016/j.ijcard.2015.03.423
Daugherty, A., Manning, M. W., & Cassis, L. A. (2000). Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. The Journal of clinical investigation, 105, 1605–1612. https://doi.org/10.1172/jci7818
Chen, T., Jiang, N., Zhang, S., Chen, Q., & Guo, Z. (2021). BAPN-induced rodent model of aortic dissecting aneurysm and related complications. Journal of thoracic disease, 13, 3643–3651. https://doi.org/10.21037/jtd-21-605
Ren, W., Liu, Y., Wang, X., Jia, L., Piao, C., Lan, F., & Du, J. (2016). β-Aminopropionitrile monofumarate induces thoracic aortic dissection in C57BL/6 mice. Scientific reports, 6, 28149. https://doi.org/10.1038/srep28149
Pereira, L., Andrikopoulos, K., Tian, J., Lee, S. Y., Keene, D. R., Ono, R., Reinhardt, D. P., Sakai, L. Y., Biery, N. J., Bunton, T., et al. (1997). Targeting of the gene encoding fibrillin-1 recapitulates the vascular aspect of Marfan syndrome. Nature genetics, 17, 218–222. https://doi.org/10.1038/ng1097-218
Pereira, L., Lee, S. Y., Gayraud, B., Andrikopoulos, K., Shapiro, S. D., Bunton, T., Biery, N. J., Dietz, H. C., Sakai, L. Y., & Ramirez, F. (1999). Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proceedings of the National Academy of Sciences of the United States of America, 96, 3819–3823. https://doi.org/10.1073/pnas.96.7.3819
Judge, D. P., Biery, N. J., Keene, D. R., Geubtner, J., Myers, L., Huso, D. L., Sakai, L. Y., & Dietz, H. C. (2004). Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. The Journal of clinical investigation, 114, 172–181. https://doi.org/10.1172/jci20641
Zhang, W. M., Liu, Y., Li, T. T., Piao, C. M., Liu, O., Liu, J. L., Qi, Y. F., Jia, L. X., & Du, J. (2016). Sustained activation of ADP/P2ry12 signaling induces SMC senescence contributing to thoracic aortic aneurysm/dissection. Journal of molecular and cellular cardiology, 99, 76–86. https://doi.org/10.1016/j.yjmcc.2016.08.008
Love, M. I., Huber, W., & Anders, S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome biology, 15, 550. https://doi.org/10.1186/s13059-014-0550-8
Wang, X., & Khalil, R. A. (2018). Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Advances in pharmacology (San Diego, Calif), 81, 241–330. https://doi.org/10.1016/bs.apha.2017.08.002
Edwards, D. R., Handsley, M. M., & Pennington, C. J. (2008). The ADAM metalloproteinases. Molecular aspects of medicine, 29, 258–289. https://doi.org/10.1016/j.mam.2008.08.001
Kelwick, R., Desanlis, I., Wheeler, G. N., & Edwards, D. R. (2015). The ADAMTS (A disintegrin and metalloproteinase with thrombospondin motifs) family. Genome biology, 16, 113. https://doi.org/10.1186/s13059-015-0676-3
Reiser, J., Adair, B., & Reinheckel, T. (2010). Specialized roles for cysteine cathepsins in health and disease. The Journal of clinical investigation, 120, 3421–3431. https://doi.org/10.1172/jci42918
Pedroza, A. J., Tashima, Y., Shad, R., Cheng, P., Wirka, R., Churovich, S., Nakamura, K., Yokoyama, N., Cui, J. Z., Iosef, C., et al. (2020). Single-cell transcriptomic profiling of vascular smooth muscle cell phenotype modulation in Marfan syndrome aortic aneurysm. Arteriosclerosis, thrombosis, and vascular biology, 40, 2195–2211. https://doi.org/10.1161/atvbaha.120.314670
Jin, S., Guerrero-Juarez, C. F., Zhang, L., Chang, I., Ramos, R., Kuan, C. H., Myung, P., Plikus, M. V., & Nie, Q. (2021). Inference and analysis of cell-cell communication using Cell Chat. Nature communications, 12, 1088. https://doi.org/10.1038/s41467-021-21246-9
Golovina, V. A., & Blaustein, M. P. (2006). Preparation of primary cultured mesenteric artery smooth muscle cells for fluorescent imaging and physiological studies. Nature protocols, 1, 2681–2687. https://doi.org/10.1038/nprot.2006.425
Desbois, M., Udyavar, A. R., Ryner, L., Kozlowski, C., Guan, Y., Dürrbaum, M., Lu, S., Fortin, J. P., Koeppen, H., Ziai, J., et al. (2020). Integrated digital pathology and transcriptome analysis identifies molecular mediators of T-cell exclusion in ovarian cancer. Nature communications, 11, 5583. https://doi.org/10.1038/s41467-020-19408-2
Stenmark, K. R., Davie, N., Frid, M., Gerasimovskaya, E., & Das, M. (2006). Role of the adventitia in pulmonary vascular remodeling. Physiology (Bethesda, Md), 21, 134–145. https://doi.org/10.1152/physiol.00053.2005
Tillie, R., van Kuijk, K., & Sluimer, J. C. (2020). Fibroblasts in atherosclerosis: Heterogeneous and plastic participants. Current opinion in lipidology, 31, 273–278. https://doi.org/10.1097/mol.0000000000000700
Holm, T. M., Habashi, J. P., Doyle, J. J., Bedja, D., Chen, Y., van Erp, C., Lindsay, M. E., Kim, D., Schoenhoff, F., Cohn, R. D., et al. (2011). Noncanonical TGFβ signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science (New York, NY), 332, 358–361. https://doi.org/10.1126/science.1192149
Künzel, S. R., Hoffmann, M., Weber, S., Künzel, K., Kämmerer, S., Günscht, M., Klapproth, E., Rausch, J. S. E., Sadek, M., Kolanowski, T., et al. (2021). Diminished PLK2 induces cardiac fibrosis and promotes atrial fibrillation. Circulation research. https://doi.org/10.1161/circresaha.121.319425
Xie, Z., Singh, M., & Singh, K. (2004). ERK1/2 and JNKs, but not p38 kinase, are involved in reactive oxygen species-mediated induction of osteopontin gene expression by angiotensin II and interleukin-1beta in adult rat cardiac fibroblasts. Journal of cellular physiology, 198, 399–407. https://doi.org/10.1002/jcp.10419
Yu, H. W., Liu, Q. F., & Liu, G. N. (2010). Positive regulation of the Egr-1/osteopontin positive feedback loop in rat vascular smooth muscle cells by TGF-beta, ERK, JNK, and p38 MAPK signaling. Biochemical and biophysical research communications, 396, 451–456. https://doi.org/10.1016/j.bbrc.2010.04.115
Lin, Y. H., Huang, C. J., Chao, J. R., Chen, S. T., Lee, S. F., Yen, J. J., & Yang-Yen, H. F. (2000). Coupling of osteopontin and its cell surface receptor CD44 to the cell survival response elicited by interleukin-3 or granulocyte-macrophage colony-stimulating factor. Molecular and cellular biology, 20, 2734–2742. https://doi.org/10.1128/mcb.20.8.2734-2742.2000
Barczyk, M., Carracedo, S., & Gullberg, D. (2010). Integrins. Cell and tissue research, 339, 269–280. https://doi.org/10.1007/s00441-009-0834-6
Müller, U., Wang, D., Denda, S., Meneses, J. J., Pedersen, R. A., & Reichardt, L. F. (1997). Integrin alpha8beta1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell, 88, 603–613. https://doi.org/10.1016/s0092-8674(00)81903-0
Littlewood Evans, A., & Müller, U. (2000). Stereocilia defects in the sensory hair cells of the inner ear in mice deficient in integrin alpha8beta1. Nature genetics, 24, 424–428. https://doi.org/10.1038/74286
Lin, Z., Tian, X. Y., Huang, X. X., He, L. L., & Xu, F. (2019). microRNA-186 inhibition of PI3K-AKT pathway via SPP1 inhibits chondrocyte apoptosis in mice with osteoarthritis. Journal of cellular physiology, 234, 6042–6053. https://doi.org/10.1002/jcp.27225
Zeng, B., Zhou, M., Wu, H., & Xiong, Z. (2018). SPP1 promotes ovarian cancer progression via Integrin β1/FAK/AKT signaling pathway. OncoTargets and therapy, 11, 1333–1343. https://doi.org/10.2147/ott.s154215
Zheng, Y. H., Tian, C., Meng, Y., Qin, Y. W., Du, Y. H., Du, J., & Li, H. H. (2012). Osteopontin stimulates autophagy via integrin/CD44 and p38 MAPK signaling pathways in vascular smooth muscle cells. Journal of cellular physiology, 227, 127–135. https://doi.org/10.1002/jcp.22709
Parsons, C. J., Takashima, M., & Rippe, R. A. (2007). Molecular mechanisms of hepatic fibrogenesis. Journal of gastroenterology and hepatology, 22(Suppl 1), S79-84. https://doi.org/10.1111/j.1440-1746.2006.04659.x
Babicheva A, Makino A, Yuan JX. (2021) mTOR signaling in pulmonary vascular disease: Pathogenic role and therapeutic target. International Journal of Molecular Sciences 22https://doi.org/10.3390/ijms22042144
Wang, S. K., Green, L. A., Gutwein, A. R., Gupta, A. K., Babbey, C. M., Motaganahalli, R. L., Fajardo, A., & Murphy, M. P. (2018). Osteopontin may be a driver of abdominal aortic aneurysm formation. Journal of vascular surgery, 68, 22s–29s. https://doi.org/10.1016/j.jvs.2017.10.068
O’Brien, E. R., Garvin, M. R., Stewart, D. K., Hinohara, T., Simpson, J. B., Schwartz, S. M., & Giachelli, C. M. (1994). Osteopontin is synthesized by macrophage, smooth muscle, and endothelial cells in primary and restenotic human coronary atherosclerotic plaques. Arteriosclerosis and thrombosis: A journal of vascular biology, 14, 1648–1656. https://doi.org/10.1161/01.atv.14.10.1648
Coskun S, Atalar E, Ozturk E, Yavuz B, Ozer N, Goker H, Ovünç K, Aksöyek S, Kes S, Sivri B et al. (2006) Plasma osteopontin levels are elevated in non-ST-segment elevation acute coronary syndromes. Journal of the National Medical Association 98:1746–1750.
Wolak, T. (2014). Osteopontin - a multi-modal marker and mediator in atherosclerotic vascular disease. Atherosclerosis, 236, 327–337. https://doi.org/10.1016/j.atherosclerosis.2014.07.004
Lorenzen, J. M., Neunhöffer, H., David, S., Kielstein, J. T., Haller, H., & Fliser, D. (2010). Angiotensin II receptor blocker and statins lower elevated levels of osteopontin in essential hypertension–results from the EUTOPIA trial. Atherosclerosis, 209, 184–188. https://doi.org/10.1016/j.atherosclerosis.2009.09.009
Acknowledgements
We thank Prof. Michael P. Fischbein for the aortic scRNA-seq data of Marfan Syndrome mice and Prof. Dongjin Wang for helping prepare the human aortic aneurysm and arterial tissue samples
Funding
This work was supported by the National Natural Science Foundation of China (81770250, 81930014, 81770470) and the Beijing Young Top-notch Talent Program (2017000021223ZK35).
Author information
Authors and Affiliations
Contributions
MZ conceptualized the study, performed experiments, carried out the analyses, and drafted the initial manuscript. YZ performed data analysis. ZZ, FQ, and SZ conducted samples and a part of data collection. SG and YuL supervised the analyses. JD and YaL participated in the study design, reviewed, and revised the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All animal experiments were performed in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals, and the protocols were approved by the Institutional Animal Care and Use Committee of Capital Medical University. Use of human samples was approved by the Medical Ethical Committee of Beijing Anzhen Hospital, Capital Medical University and Affiliated Drum Tower Hospital of Nanjing University Medical School and in concordance with the principles outlined in the Declaration of Helsinki. Informed consent was obtained.
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, M., Zhu, Y., Zhou, Z. et al. Fibroblast-Secreted Phosphoprotein 1 Mediates Extracellular Matrix Deposition and Inhibits Smooth Muscle Cell Contractility in Marfan Syndrome Aortic Aneurysm. J. of Cardiovasc. Trans. Res. 15, 959–970 (2022). https://doi.org/10.1007/s12265-022-10239-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-022-10239-8