Abstract
Understanding the cardiac-coronary interaction is fundamental to developing treatment strategies for ischemic heart disease. We sought to examine the impact of afterload reduction following isosorbide dinitrate (ISDN) administration on LV properties and coronary hemodynamics to further our understanding of the cardiac-coronary interaction. Novel methodology enabled real-time simultaneous acquisition and analysis of coronary and LV hemodynamics in vivo using coronary pressure-flow wires (used to derive coronary wave energies) and LV pressure-volume loop assessment. ISDN administration resulted in afterload reduction, reduced myocardial demand, and increased mechanical efficiency (all P<0.01). Correlations were demonstrated between the forward compression wave (FCW) and arterial elastance (r=0.6) following ISDN. In the presence of minimal microvascular resistance, coronary blood flow velocity exhibited an inverse relationship with LV elastance. In summary this study demonstrated a reduction in myocardial demand with ISDN, an inverse relationship between coronary blood flow velocity and LV contraction-relaxation and a direct correlation between FCW and arterial elastance.
Graphical abstract
The pressure volume-loop and corresponding parameters b The pressure volume loop before (solid line) and after (broken line) Isosorbide dintrate

Similar content being viewed by others
Introduction
An understanding of the cardiac-coronary interaction is fundamental to the development of treatment strategies in ischemic heart disease [1, 2]. Advances in technology have enabled invasive measurement of left ventricular mechanics to be performed safely in humans. Invasive ventricular pressure-volume (PV) analysis is the current gold standard clinical research method for assessment of left ventricular contractile function and for measuring LV afterload (Fig. 1a) [1, 3]. LV elastance measured using PV analysis has been shown to provide a more accurate measure of contractile properties than conventional indices [4]. Arterial elastance (Ea) provides a more representative measure of LV afterload as it combines both steady and pulsatile forces on the LV and can be derived from the PV loop as the ratio of LV end-systolic pressure to stroke volume [5]. Unlike mean arterial pressure, Ea is not routinely measured in clinical practice, however, this invasive measure of afterload will enable us to monitor the impact of afterload reduction on peripheral and coronary vasculature more accurately. Coronary wave intensity analysis (WIA) derived from coronary pressure and flow velocity can help further identify the transmission of such forces on coronary blood flow by separating the transmission of energy into forward and backward waveforms [6].
An example of data analysis using the PV loop and acquisition with in-vivo fluoroscopic imaging and ex-vivo demonstration of instrumentation. a The pressure-volume (PV) loop and derived measurements. The pressure-volume loop describes a single cardiac cycle by presenting left ventricular pressure as function of volume and facilitates an understanding of cardiac hemodynamics. The end-systolic pressure-volume relationship (ESPVR) line slope (or Ees) represents the load-independent contractile function of the heart. The end-diastolic pressure-volume relationship (EDPVR) line slope (EDPVR) represents the load-independent diastolic properties of the heart. The area of the pressure-volume loop (gray area) represents stroke work (SW), the combination of SW and potential energy (boundary created from the ESPVR and EDPVR lines) represents what is referred to pressure-volume area (PVA or total cardiac work), and arterial elastance is the ratio of end-systolic pressure to stroke volume (Ea). b In vivo fluoroscopic image of real-time simultaneous intracoronary and intraventricular data acquisition. Fluoroscopy was used to confirm (PA view) correct positioning of Combowire in the coronary artery (left anterior descending) and conductance catheter (CC) in the apex of the left ventricle. c Ex vivo image of Asahi sheathless guide with CC and Combowire exiting the guide catheter
The dominant effects of nitrates in the presence of coronary artery disease are believed to be as a result of vasodilatation secondary to afterload reduction [7]. Conclusions are limited however due to the use of surrogate measures of left ventricular mechanics and the assessment of either coronary or left ventricular forces in isolation. To overcome previous limitations, we devised novel methodology, enabling simultaneous invasive assessment of both LV and coronary hemodynamics for the first time. In this physiological study, we sought to examine the impact of afterload reduction following isosorbide dinitrate (ISDN) administration on left ventricular properties and coronary hemodynamics to further our understanding of the cardiac-coronary interaction. The aim of the study was to better understand the relationship between cardiac contraction and coronary blood flow before and after ISDN administration and how the reduction in afterload translates physiologically to anti-anginal properties.
Methods
Study Population
This prospective study was conducted at St Thomas Hospital, London, UK. Participants were identified from waiting lists for coronary angiography. Eligibility was confirmed following coronary angiography with identification of coronary artery disease in one or more vessels. This was defined as greater than 50% diameter stenosis in two orthogonal angiographic views. Exclusion criteria included previous coronary artery bypass graft surgery, severe aortic stenosis, and chronic total occlusions that prevented simultaneous LV or coronary hemodynamic measurements. Patients with moderate to severe LV impairment (ejection fraction <45%) were excluded due to a lack of contractile reserve. Participants were requested to stop all oral vasodilator preparations 72 h prior. All patients underwent written, informed consent prior to partaking in the study. This study was deemed safe and received approval from the National Research Ethics Committee (08/H0802/136). In this cohort, 12 patients were necessary to examine the impact of afterload reduction (end-systolic pressure) on cardiac-coronary interaction, providing 25mmHg mean difference (significance defined by two sided alpha 0.05, 80% power). Following interim data review, a further 3 patients were recruited to maximize power due to higher than expected variance about the mean.
Experimental Protocol and Instrumentation
Left heart catheterization was performed via the right radial artery (6Fr). Following angiographic identification of coronary stenosis, within the same sitting, patients were entered into the study (to avoid multiple procedures), and weight adjusted heparin was administered (70IU/kg) intra-arterially. Thereafter, an 80cm 7.5Fr sheathless guide catheter was used to introduce a dual-sensor pressure-velocity 0.014” coronary guide wire (Combowire, Philips Del Mar) distal to the stenosis in the target coronary artery. The guide catheter was disengaged from the coronary ostium, and a 4Fr conductance catheter (CC) (CD Leycom, The Netherlands) was delivered to the ventricular cavity across the aortic valve via the same guide catheter (Fig. 1b and c). Correct positioning was confirmed by fluoroscopy (Fig. 1b) and conductance signals. Median time from initial diagnostic angiography to completion of instrumentation and initiation of data acquisition was 12 min. At this point, patients were administered either isosorbide dinitrate (ISDN). Baseline measurements including coronary pressure and flow and LV pressure-volume were performed in all patients, and patients acted as their own control. Afterload reduction was performed by manual injection of 1mg (diluted in 10 ml) isosorbide dinitrate through the manifold into the aortic root. Continuous measurements of LV pressure and volume and coronary pressure and flow velocity were performed. Variables were averaged from 5 cardiac cycles. Following the ISDN experimental protocol, physiological assessment of the coronary stenosis was performed with intravenous adenosine (140 mcg/kg/min) peripheral infusion. This was to determine the functional significance of the coronary artery lesion at maximal, steady state hyperemia (maximal coronary blood flow velocity), and patients underwent angioplasty where necessary.
In order to examine the direct relationship between LV contraction-relaxation and coronary flow impediment (compression-relaxation) through a single cardiac cycle, adenosine was also to abolish autoregulatory processes to maximize coronary and capillary vasodilatation. As above continuous recording of resting, simultaneous coronary and LV PV loop measurements were performed at baseline and following the above dosing regimen of IV adenosine administered for 120 s until maximal hyperaemia was achieved. This data was used to generate LV elastance—flow loops (cardiac-coronary interaction plots) and elastance-pressure loops as described below.
Data Acquisition and Analysis
Simultaneous data acquisition and analysis are described in the supplemental methods [2]. Coronary pressure and flow velocity signals (Combomap system, Volcano Corp) and CC measurements were acquired in real time (Conduct NT, version 3.18.1 CD Leycom). Mean coronary blood flow velocity (U) and distal coronary pressure (Pd) were determined from the Doppler signal and high-fidelity pressure signal, respectively, distal to the coronary stenosis. Figure 1a demonstrates the pressure-volume (PV) loop, and corresponding points on the loop that data were acquired. End-diastole was measured at the peak of the R wave on the ECG, and end-systole was measured at the point of maximum pressure/volume in the cardiac cycle. End-diastolic (EDV) and end-systolic volumes (ESV) were measured from the PV loop. Stroke volume was calculated as the difference in these volumes. End-systolic (ESP) and end-diastolic pressures (EDP) were also measured directly from the PV loop. These baseline measurements were then used to calculate various parameters related to LV performance. The end-systolic pressure-volume relationship curve has previously been shown to be independent of volume loading, within physiological range, and is the most accurate measure of LV contractile function.
This was calculated as the ratio of ESP to ESV, referred to as Ees (single point, SP) [8]. The volume-axis intercept (Vo) of the ESPVR was assumed to be 0 (IVC occlusion was not performed). The slope of the end-diastolic pressure-volume relationship was used to describe LV diastolic function and was calculated using the following equation EDP = An.EDV (Bn), with An = 28.2 mmHg and Bn = 2.79; the values are expressed as the alpha (An) and beta (Bn) coefficients of the equation [9]. LV elastance is the LV pressure-volume relationship over one cardiac cycle. It is a function of LV contractile function over time from relaxation to contraction this forms a bell-shaped curve, where either end (troughs) of the curve represents the end-diastolic pressure-volume relationship (minimum LV pressure) and the peak of the curve represents the end-systolic pressure relationship (ESPVR). In this manuscript, the term LV elastance is used interchangeably to describe contractile function.
LV stroke work which is the external work produced by the ventricle was calculated from the area of the PV loop (Fig. 1a).
Effective arterial elastance (Ea) is the ratio of end-systolic pressure to stroke volume and combines the steady and pulsatile components of left ventricular afterload. Pressure-volume area (PVA) represents total cardiac work and was estimated as the sum of stroke work (SW) and elastic potential energy (Fig. 1a) and has previously been demonstrated to be linearly proportional to myocardial oxygen consumption [10]. LV mechanical efficiency was determined by the ratio of effective stroke work to overall cardiac work (SW/PVA) expressed as a percentage.
For the purposes of analysis, wave intensity analysis (WIA) was performed to establish the temporal relationship between the main coronary wave energies and the cardiac cycle. Wave intensity analysis (WIA) was performed using time derivatives obtained after smoothing the raw signals using an adaptive Savitzky-Golay filter to improve WIA robustness, which has previously been described [11, 12]. Net wave intensity (dI) was performed and normalized to the sampling rate, and separation into forward and backward components was performed using the single-point technique [13]. Baseline pulse wave velocity was implemented for WIA determination during nitrate administration and following adenosine to account for limitations in the single-point technique during hyperemia [14]. We examined the association between afterload and the forward compression wave (FCW), the timing of which has previously been associated with peak aortic pressure, responsible for systolic acceleration of coronary blood flow. We also examined the relationship between diastolic blood pressure and the forward expansion wave which causes late systolic deceleration following a fall in aortic pressure. All four main waves were included in the temporal analysis.
Statistical Analysis
Quantitative data are expressed as mean (SD) and median (IQR); categorical variables are described as proportions and percentages. Data were assessed for normality of (Gaussian) distribution both graphically and by use of the Shapiro-Wilk test. Statistical comparison of serial hemodynamic measurements (quantitative data) of normal distribution within subjects was performed using paired t-tests. Those not of normal distribution were analyzed using Wilcoxon signed rank test. Categorical data were compared by use of the Pearson chi-square test. Correlations were assessed using Pearson’s correlation coefficient. A P value of <0.05 was considered statistically significant for all tests. Statistical analysis was performed using SPSS v24.0 (IBM).
Results
Between December 2013 and May 2016, 15 patients completed the study protocol. Baseline and procedural characteristics of the study population are provided in Table 1. Twenty patients were enrolled, 2 patients had only minor atheroma on coronary angiography, and 3 patients could not complete the study protocol. Baseline measurements were performed in all patients, and patients served as their own control. Eleven of the fifteen patients demonstrated flow-limiting (FL) coronary artery lesions (median FFR 0.72; IQR 0.70 to 0.79). Four of the fifteen patients did not have flow-limiting (NFL) coronary lesions (median FFR 0.88; IQR 0.86 to 0.96) following adenosine administration. All patients responded similarly to ISDN, and there were no differences in baseline characteristics or hemodynamics, details of which and comparison between cohorts (functionally significant versus non-significant lesions) is provided as a table in the supplemental material. Patients were therefore analyzed together and acted as their own control.
Left Ventricular Hemodynamics
Left ventricular hemodynamic measurements at baseline and following ISDN are presented in Table 2. Graphical representation of LV and coronary hemodynamic measurements are presented in Fig. 2a–g. Administration of intra-arterial ISDN resulted in a leftward, downward shift of the pressure-volume loop compared to baseline (Fig. 2i). Administration of intra-arterial ISDN resulted in a reduction in left ventricular pressures (end-diastolic pressure 8.3±6.4 versus 13.1±6.2mmHg, P<0.001; end-systolic pressure 96.0±19.0 versus 122.7±18.0mmHg, P<0.001) and left ventricular volumes (end-diastolic volume 102.7±129.5 versus 112.1±32.7mL, P=0.01, end-systolic volume 36.1±15.6 versus 44.3±15.3mL, P=0.04) compared to baseline. On administration of intra-arterial ISDN, there was a reduction in afterload (arterial elastance 1.3±0.5 versus 1.6±0.5 mmHg/mL, P=0.003) and a reduction in stroke work (6348±2670 versus 7243±2637mmHg.mL, P=0.002) and pressure-volume area (1.15±0.4 versus 1.49±0.56J, P<0.001) compared to baseline. Administration of intra-arterial ISDN resulted in increased LV mechanical efficiency as a ratio of stroke work to pressure-volume area (73.8±9.0 versus 65.2±6.3%, P=0.001). There was also an improvement in the active diastolic properties of the ventricle (Tau 32.5±4.3 versus 34.1±4.6ms; P=0.038) but a decrease in magnitude of load-dependent markers of diastolic function (dP/dTmin −906±355 versus −1284±179mmHg/s; P=0.011).
Graphical demonstration of changes induced by ISDN and exercise. a–g Heart rate, ST segment, power output, EDP, EDP dPdtmax, and coronary flow velocity changes from baseline following administration of ISDN or dynamic exercise. Measurements are at baseline, 50% peak, and peak effect. H LV pressure-volume relations at baseline (black solid line), 50% peak (broken line), and peak exercise (solid red line). Peak exercise effect is associated with increased myocardial demand, stroke volume, and LV pressures. i LV pressure-volume relations at baseline (black solid line), 50% peak (broken line), and peak ISDN effect (solid red line). Peak ISDN effect is consistent with a decrease in stroke work, increase in LV efficiency, and reduction in myocardial oxygen consumption
Coronary Hemodynamics and Wave Intensity Analysis
Prior to ISDN administration median resting PdPa was 0.83 (IQR 0.67 to 0.95). ISDN administration resulted in a significant decrease in distal coronary pressure (76 versus 87mmHg; P=0.003), microvascular resistance (5.4 versus 7.4 mmHg/cm/s; P<0.001), and stenosis resistance (2.4 versus 3.9 mmHg/cm/s; P<0.001) compared to baseline with an increase in diastolic time fraction (0.68 versus 0.64; P=0.002) compared to baseline. Wave intensity analysis demonstrated no significant changes in the magnitude of coronary wave energies following administration of intra-arterial ISDN; however, there was a near significant numerical reduction in FCW energy (5822 versus 4568 J/mmHg/s; P=0.06).
Simultaneous Analysis
Figure 3a provides a visual depiction and representative example of simultaneous coronary and LV hemodynamic measurements following intra-arterial ISDN administration as a function of time; a simultaneous drop in LV pressure and subtle increase in coronary blood flow velocity can be seen at 15–20 s. Figure 3b demonstrates the same hemodynamic measurements averaged over one cardiac cycle with coronary wave energies at baseline and following ISDN.
Simultaneous coronary and LV data acquisition. a Representative example of simultaneous coronary and LV hemodynamic measurements following intra-arterial ISDN administration as a function of time. From top to bottom, the panels display continuous intra-cardiac ECG recording, left ventricular pressure (LVP), left ventricular volume (LVV), distal coronary pressure (Pd), and mean coronary flow velocity (U). b demonstrates simultaneous coronary and LV hemodynamic measurements at baseline and following intra-arterial ISDN administration averaged over one cardiac cycle with coronary wave energies at baseline and following ISDN. c demonstrates simultaneous coronary and LV hemodynamic measurements at baseline and during exercise averaged over one cardiac cycle and coronary wave energies at baseline and during dynamic exercise
To examine the association between coronary wave energies and left ventricular pressure-volume, we mapped the timing of the origin and termination of wave energies to the cardiac cycle as measured by the pressure-volume loop during left ventricular contraction and relaxation during resting conditions (Fig. 4). We describe the four main wave energies responsible for acceleration and deceleration of coronary blood flow in relation to the timing of LV contraction and relaxation (Fig. 4b and c). The forward compression wave (FCW) was shown to be generated during isovolumic contraction just prior to aortic valve opening and terminated at peak left ventricular pressure (LV pressure equal to aortic systolic pressure). The backward expansion wave (BEW) was shown to be generated at end-systole (aortic valve closure) and terminated near end-diastole, at the point of minimum left ventricular pressure. The backward compression wave was generated during isovolumic contraction and terminated on aortic valve opening. The forward expansion wave (FEW) was initiated at maximal LV contraction at the point of peak systolic blood pressure and terminated at end-systole (when LV pressure is equal to diastolic blood pressure). The timings of the coronary wave energies including initiation, termination, and peak are presented alongside timings of the major events in the cardiac cycle in Fig. 4d. Wave timings did not change with either exercise or ISDN administration.
Coronary wave energies superimposed on the pressure-volume (PV) loop. a The cardiac cycle depicted as LV pressure (LVP) as function of volume (LVV), the end-diastole (ED), end-systole (ES), and dP/dT max and dP/dTmin (maximal rate of pressure increase and pressure decline, respectively) depicted on the PV loop. b The forward travelling compression wave (FCW) in the coronary artery originates when LV pressure equals aortic diastolic pressure (immediately prior to aortic valve opening) and terminates immediately prior to peak LV pressure. The forward expansion wave (FEW) originates when LV pressure equals aortic systolic pressure (peak LV pressure) and terminates during isovolumic relaxation at the point of dP/dT min (maximal LV rate of relaxation). c The backward compression wave (BCW) originates during isovolumic contraction and terminates immediately after aortic valve opening (when LV pressure exceeds aortic diastolic pressure). The backward expansion wave (BEW) originates on aortic valve closure, at end-systole, and terminates at minimum LV pressure. d Wave generation during the cardiac cycle. The black broken line represents end-systole, with standard deviation (red broken line). ED end-diastole, BCW backward compression wave, FCW forward compression wave, BEW backward expansion wave, ES end-systole, FEW forward expansion wave, ms milliseconds, s start, p peak, e end
In light of the above findings and the potential relationship between FCW with aortic pressure, we sought to examine the relationship between the FCW with LV afterload as measured by arterial elastance in the ISDN cohort at baseline. On simultaneous analysis of PV loop and coronary wave energies, afterload as measured by arterial elastance strongly correlated with forward compression wave energy in the coronary artery in all patients at baseline (r=0.6, P=0.004) and is graphically depicted in Fig. 5a. This relationship was maintained following ISDN administration. Increasing lesions severity was associated with increased magnitude of FCW energy. Furthermore, we examined the relationship between diastolic blood pressure and FEW energy; on simultaneous analysis, we also demonstrated a correlation between FEW magnitude and measured diastolic blood pressure (r=0.6, P=0.001).
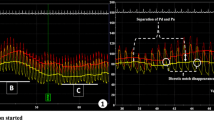
We then examined the association between left ventricular elastance (contractile properties) and coronary blood flow velocity through one cardiac cycle. Figure 6a demonstrates LV elastance over time through one cardiac cycle at baseline. In Fig. 6b, we plotted coronary blood flow velocity against LV elastance at baseline. This demonstrated a figure of eight curve with coronary flow velocity peaking with minimal elastance, and Fig. 6c demonstrates distal coronary pressure as a transmission of LV elastance. Following administration of IV adenosine, to minimize microvascular resistance, there was an increase in LV elastance (Fig. 6d), and in Fig. 6e, we demonstrate a clear inverse exponential relationship between coronary blood flow velocity and LV elastance.
Coronary hemodynamics as a function of LV elastance over a single cardiac cycle. In this diagram, we relate cardiac contraction to coronary blood flow velocity and coronary pressure. a to c are under resting/baseline conditions. Cardiac contraction over one cardiac cycle is represented as elastance (instantaneous LV pressure/volume). This generates a bell-shaped curve seen in a. This starts and ends at end-diastole when LV pressure is minimal and peaks in the center at end-systole. The aim of this plot is to represent LV contraction and relaxation over one cardiac cycle. b This is a simultaneous plot of LV elastance (in a) against coronary blood flow velocity to demonstrate how LV contraction-relaxation impacts coronary flow velocity; this is performed under resting, baseline conditions. c This is a simultaneous plot of LV elastance (from a) against distal coronary pressure measured by the Combowire, to demonstrate how LV contraction-relaxation affects coronary artery pressure. d–f are the same plots after IV adenosine administration; the aim is to abolish autoregulatory processes and maximize vasodilatation to see how this changes the cardiac-coronary interaction D LV elastance plotted against time following adenosine E simultaneous plot of LV elastance (in a) against coronary blood flow velocity to demonstrate how LV contraction-relaxation impacts coronary flow velocity following IV adenosine F simultaneous plot of LV elastance (from a) against distal coronary pressure measured by the Combowire, to demonstrate how LV contraction-relaxation affects coronary artery pressure. U coronary blood flow velocity, ED end-diastole, ED+10 end-diastole + 10ms to identify direction of figure of 8 graph over time, ES end-systole
Discussion
This study methodology enabled simultaneous analysis of coronary hemodynamics and left ventricular pressure-volume parameters. The aim of the study was to better understand the relationship between cardiac contraction and coronary blood flow before and after ISDN administration and how the reduction in afterload translates physiologically to anti-anginal properties. Furthermore, we sought to examine the direct relationship between LV contraction-relaxation and coronary flow (compression-relaxation) during a single cardiac cycle to better understand how the proximity of LV myocardium and coronary arteries impact coronary blood flow. Adenosine was then used to minimize microvascular resistance so that we could identify a direct relation between the two. The main findings of the study are as follows: (1) In the presence of minimal microvascular resistance, coronary blood flow velocity exhibits a predominantly linear inverse relationship with LV contraction through one cardiac cycle. Relative to baseline, the relationship between coronary flow velocity and LV contraction is shifted upward in the presence of minimal MVR; thus for a fixed measure of LV contractility, coronary blood flow velocity is higher. (2) ISDN reduced afterload, preload, and therefore myocardial demand with a subtle increase in coronary flow velocity due to reduction in MVR. (3) Afterload as measured by arterial elastance was shown to strongly correlate with the forward compression wave. (4) The forward compression wave energies derived from invasive coronary hemodynamics can be used as a surrogate measure of arterial elastance without the need for simultaneous invasive LV measurement.
LV elastance is the LV pressure-volume relationship over one cardiac cycle. This is visually depicted in Fig. 6a where either end of the curve represents the end-diastolic pressure-volume relationship (minimum LV pressure) and the peak of the curve represents the end-systolic pressure relationship (ESPVR). Importantly, we were able to demonstrate a relationship between coronary flow velocity and LV elastance (end-systolic pressure-volume relationship over time) for the first time. With autoregulatory processes intact, this relationship is a figure of eight curve. Following administration of IV adenosine to minimize microvascular resistance, we demonstrated a direct linear inverse relationship between coronary flow velocity and LV elastance. This relationship translates clinically to deceleration of coronary flow as the left ventricle contracts until the ventricle reaches the point of maximal contraction. Adenosine also induces maximal vasodilatation (or minimal microvascular resistance), the effect of which is to increase coronary flow, our data demonstrate an upward shift in the cardiac contraction-coronary flow interaction such that for a given measure of LV elastance, coronary flow velocity is increased (Fig. 5e), and despite an increase in contractility (demonstrated with adenosine), coronary blood flow is preserved. This mechanism can be potentially used to explain the cardioprotective effect demonstrated by adenosine and other agents which enhance cardiac contractile function while at the same time driving vasodilatation, such as in the setting of acute heart failure.
We demonstrated that administration of ISDN was associated with a decrease in preload, afterload, total cardiac work, and increased efficiency, thus reducing myocardial demand and therefore the mechanism by which ISDN provides an anti-anginal effect. This study confirmed through simultaneous measurement of coronary and LV hemodynamics that the dominant effects of ISDN were as a result of vasodilatation. ISDN resulted in significant reductions in distal coronary pressure, microvascular resistance, and stenosis resistance with subtle early increase in coronary flow velocity. The latter was likely attributable to wash down the coronary artery from intra-aortic ISDN administration.
Coronary wave intensity analysis generates forward and backward travelling waves [6, 15]. The forward waves are believed to arise upstream, although as a result of left ventricular ejection and relaxation, these waves are believed to be transmitted from the aorta to the coronary artery, thus are forward travelling. Backward travelling waves identified within the coronary artery are thought to originate directly from the myocardium and are generated from the transmission of downstream events in a retrograde manner through the coronary artery. Waves that cause a pressure increase are referred to as compression waves; waves causing a decrease in pressure are referred to as suction or expansion waves. The effect on coronary blood velocity can then be determined by both the origin (forward or backward) and the change in pressure (compression or expansion); for example, an increase in coronary blood velocity can be generated either by a forward travelling compression wave (increase in pressure) or a backward travelling expansion. In this study, administration of ISDN enabled us to vary arterial elastance and demonstrate a direct correlation between the forward compression wave (FCW, flow acceleration arising from aortic pressure) and afterload reduction. Arterial elastance is a superior measure of afterload combining steady and pulsatile forces on the left ventricle [5]; however, this cannot be routine measured in clinical practice without instrumentation of the left ventricle. Therefore, this study demonstrates that using a coronary pressure-flow wire, the derived FCW energy can be used an accurate surrogate measure of afterload and is likely to be superior to measuring non-invasive or invasive arterial pressure alone.
We found that with increasing lesion severity was associated with increased magnitude of FCW energy. Although this initially appears counterintuitive, we propose that because the FCW is responsible for flow acceleration, it would follow that flow velocity would be higher/increased turbulence in the presence of increased stenosis severity. As this a complex interplay between proximal and distal forces, we hypothesis that the presence of progressively severe epicardial stenosis leads to progressive vasodilatation of the coronary microcirculatory bed.
This study examined the timings and origin of wave energies by superimposing them on the cardiac cycle. This provided novel insights into the cardiac-coronary interaction, specifically, that forward travelling coronary waves are dependent on aortic pressure and originate from peripheral vasculature.
Limitations
This was a single-center study. The population examined were patients with stable single vessel coronary artery disease. A true control group in patients with normal coronary arteries and without risk factors for coronary disease would have provided an interesting comparator. ESPVR was calculated from a single point, not using single beat calculations; V0 was therefore presumed to be 0. Due to the associated technical challenges of simultaneous pressure-volume and coronary pressure and flow measurements, the number of participants was small.
Conclusions
This study assessed the impact of afterload reduction on LV mechanics with the current gold standard analytical tools. ISDN reduced afterload, preload, and therefore myocardial demand with a subtle increase in coronary flow velocity due to reduction in MVR. Novel findings include improved myocardial efficiency, an enhanced understanding of the cardiac-coronary interaction including the linear inverse relationship between coronary blood flow velocity and LV contraction-relaxation and a direct correlation between FCW and arterial elastance. Afterload as measured by arterial elastance was shown to strongly correlate with the forward compression wave, thus can be used as a surrogate measure of arterial elastance without the need for simultaneous invasive LV measurement. Targeting this wave-elastance relationship could be advantageous in developing treatment strategies in IHD.
Clinical Relevance
This study demonstrates that the mechanisms of ISDN that contribute to its anti-anginal properties are namely a reduction in cardiac workload and enhance myocardial efficiency. We also demonstrate that forward compression wave energy derived from coronary pressure-flow velocity wire can be used an accurate surrogate measure of afterload and is likely to be superior to measuring arterial pressure in isolation. Adenosine unmasks a linear relationship between LV elastance and coronary blood flow velocity with an upward shift in contractile-flow relations supporting a cardioprotective effect.
Abbreviations
- PV:
-
Pressure-volume
- LV:
-
Left ventricle
- WIA:
-
Wave intensity analysis
- ISDN:
-
Isosorbide dinitrate
- CC:
-
Conductance catheter
- U:
-
Mean coronary flow velocity
- Pd:
-
Distal coronary artery pressure
- EDV:
-
End-diastolic volume
- ESV:
-
End-systolic volume
- EDP:
-
End-diastolic pressure
- ESPVR:
-
End-systolic pressure-volume relationship
- EDPVR:
-
End-diastolic pressure-volume relationship
- Ea:
-
Arterial elastance
- PVA:
-
Pressure-volume area
- SW:
-
Stroke work
- BCW:
-
Backward compression wave
- FCW:
-
Forward compression wave
- BEW:
-
Backward expansion wave
- FEW:
-
Forward expansion wave
- dP/dtmax:
-
Maximal rate of pressure increase
- dP/dtmin:
-
Maximal rate of pressure decline
References
Gaemperli, O., et al. (2013). Real-time left ventricular pressure-volume loops during percutaneous mitral valve repair with the MitraClip system. Circulation, 127(9), 1018–1027. https://doi.org/10.1161/CIRCULATIONAHA.112.135061.
Patterson, T., et al. (2016). Novel Insights Into The Physiology Of Cardiac-Coronary Interaction: A first-in-human study utilising invasive real-time simultaneous measures of coronary and left ventricular hemodynamics. Journal of the American College of Cardiology, 67(13), 376.
Schreuder, J. J., et al. (1997). Left ventricular pressure-volume relationships before and after cardiomyoplasty in patients with heart failure. Circulation, 96(9), 2978–2986 [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/9386165. Accessed: Aug. 21, 2013.
Burkhoff, D., Mirsky, I., & Suga, H. (2005). Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: A guide for clinical, translational, and basic researchers. American Journal of Physiology. Heart and Circulatory Physiology, 289(2), H501–H512. https://doi.org/10.1152/ajpheart.00138.2005.
Kelly, R. P., et al. (1992). Effective arterial elastance as index of arterial vascular load in humans. Circulation, 86(2), 513–521. https://doi.org/10.1161/01.CIR.86.2.513.
Davies, J. E., et al. (2006). Evidence of a dominant backward-propagating “suction” wave responsible for diastolic coronary filling in humans, attenuated in left ventricular hypertrophy. Circulation, 113(14), 1768–1778. https://doi.org/10.1161/CIRCULATIONAHA.105.603050.
Asrress, K. N., et al. (2017). Physiology of angina and its alleviation with nitroglycerin. Circulation, 136(1), 24–34. https://doi.org/10.1161/CIRCULATIONAHA.116.025856.
Chen, C.-H., et al. (2001). Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. Journal of the American College of Cardiology, 38(7), 2028–2034. https://doi.org/10.1016/s0735-1097(01)01651-5.
Klotz, S., et al. (2006). Single-beat estimation of end-diastolic pressure-volume relationship: a novel method with potential for noninvasive application. American Journal of Physiology. Heart and Circulatory Physiology, 291(1), H403–H412. https://doi.org/10.1152/ajpheart.01240.2005.
Suga, H., (2003). Muscle mechanics and energetics : A comparative view cardiac energetics : from e max to pressure volume area, no. October 2002, pp. 580–585.
Rivolo, S., et al. (2017). Accurate and standardized coronary wave intensity analysis. IEEE Transactions on Biomedical Engineering, 64(5). https://doi.org/10.1109/TBME.2016.2593518.
Rivolo, S., Nagel, E., Smith, N. P., & Lee, J. (2014). Automatic selection of optimal Savitzky-Golay filter parameters for coronary wave intensity analysis. Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2014, 5056–5059.
Davies, J. E., et al. (2006). Use of simultaneous pressure and velocity measurements to estimate arterial wave speed at a single site in humans. American Journal of Physiology. Heart and Circulatory Physiology, 290(2), H878–H885. https://doi.org/10.1152/ajpheart.00751.2005.
Rolandi, M. C., et al. (2014). Wave speed in human coronary arteries is not influenced by microvascular vasodilation: Implications for wave intensity analysis. Basic Research in Cardiology, 109(2), 405. https://doi.org/10.1007/s00395-014-0405-1.
Parker, K. H. (2009). An introduction to wave intensity analysis. Medical & Biological Engineering & Computing, 47(2), 175–188. https://doi.org/10.1007/s11517-009-0439-y.
Acknowledgements
Cardiovascular Research Department, Guy’s and St Thomas Hospital
Funding
This study was supported by British Heart Foundation Clinical Research Training Fellowships, T Patterson (FS/14/11/30526), C Allen (FS/18/48/33745) H McConkey (FS/16/51/32365), J Joseph (FS/15/52/31587), R Williams (FS/11/90/29087), and K Asrress (FS/11/43/28760), and a National Institute for Health Research Academic Clinical Lectureship: T Patterson.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed Consent Statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Ana Barac oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 17 kb).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Patterson, T., Rivolo, S., Burkhoff, D. et al. Physiological Impact of Afterload Reduction on Cardiac Mechanics and Coronary Hemodynamics Following Isosorbide Dinitrate Administration in Ischemic Heart Disease. J. of Cardiovasc. Trans. Res. 14, 962–974 (2021). https://doi.org/10.1007/s12265-021-10112-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-021-10112-0