Abstract
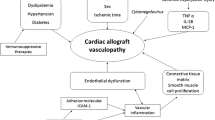
Cardiac allograft vasculopathy (CAV) is a form of accelerated atherosclerosis, which represents the leading cause of late morbidity and mortality after heart transplantation. The recent bioresorbable vascular scaffold (BVS) technology represents a potential novel therapeutic tool, in the context of CAV, by allowing transient scaffolding and concomitant vessel healing. Eligible subjects will be treated by using the Absorb Everolimus-Eluting BVS (Abbott Vascular, Santa Clara, CA, USA), and evaluated at pre-determined time points, up to 3 years since the index procedure. Both clinical and imaging data will be collected in dedicated case report forms (CRF). All imaging data will be analyzed in an independent core laboratory. The primary aim of the study is to evaluate the angiographic performance at 1 year of second-generation Absorb BVS, in heart transplant recipients affected by CAV.

Similar content being viewed by others
Abbreviations
- BVS:
-
Bioresorbable vascular scaffold
- CAD:
-
Coronary artery disease
- CAV:
-
Cardiac allograft vasculopathy
- CCS:
-
Canadian Cardiovascular Society Grading Score
- CRF:
-
Case report form
- DS:
-
Diameter stenosis
- TLR:
-
Target lesion revascularization
- EEM:
-
External elastic membrane
- FU:
-
Follow-up
- HTx:
-
Heart transplant
- ISHLT:
-
International Society for Heart & Lung Transplantation
- IVUS:
-
Intravascular ultrasound
- IVUS-VH:
-
Intravascular ultrasound-virtual histology
- MI:
-
Myocardial infarction
- MLD:
-
Minimal lumen diameter
- NTVR:
-
Non-target vessel revascularization
- OCT:
-
Optical coherence tomography
- PCI:
-
Percutaneous coronary intervention
- PDLLA:
-
Poly-d,l-lactide acid
- PLLA:
-
Poly-l-lactic acid
- RVD:
-
Reference vessel diameter
- QCA:
-
Quantitative coronary angiography
- QMI:
-
Q wave myocardial infarction
- TVR:
-
Target vessel revascularization
- VRT:
-
Vascular reparative therapy
References
Taylor, D. O., Edwards, L. B., Aurora, P., Christie, J. D., Dobbels, F., Kirk, R., et al. (2008). Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult heart transplant report—2008. The Journal of Heart and Lung Transplantation, 27(9), 943–56.
Colvin-Adams, M., Harcourt, N., & Duprez, D. (2013). Endothelial dysfunction and cardiac allograft vasculopathy. Journal of Cardiovascular Translational Research, 6(2), 263–77.
van den Hoogen, P., Huibers, M. M. H., Sluijter, J. P. G., & de Weger, R. A. (2015). Cardiac allograft vasculopathy: a donor or recipient induced pathology? Journal of Cardiovascular Translational Research, 8(2), 106–16. Springer US.
Mehra, M. R., Crespo-Leiro, M. G., Dipchand, A., Ensminger, S. M., Hiemann, N. E., Kobashigawa, J. A., et al. (2010). International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy—2010. HEALUN, 29(7), 717–27. Elsevier.
Kobashigawa, J. A., Tobis, J. M., Starling, R. C., Tuzcu, E. M., Smith, A. L., Valantine, H. A., et al. (2005). Multicenter intravascular ultrasound validation study among heart transplant recipients. Journal of the American College of Cardiology, 45(9), 1532–7.
Tomai, F., Adorisio, R., De Luca, L., Pilati, M., Petrolini, A., Ghini, A. S., et al. (2014). Coronary plaque composition assessed by intravascular ultrasound virtual histology: association with long-term clinical outcomes after heart transplantation in young adult recipients. Catheterization and Cardiovascular Interventions, 83(1), 70–7.
Tomai, F., De Luca, L., Petrolini, A., Di Vito, L., Ghini, A. S., Corvo, P. (2015). Optical coherence tomography for characterization of cardiac allograft vasculopathy in late survivors of pediatric heart transplantation. The Journal of Heart and Lung Transplantation. 0(0) Elsevier.
Hou, J., Lv, H., Jia, H., Zhang, S., Xing, L., Liu, H., et al. (2012). OCT assessment of allograft vasculopathy in heart transplant recipients. JCMG Elsevier Incorporation, 5(6), 662–3.
Authors/Task Force Members, Windecker, S., Kolh, P., Alfonso, F., Collet, J.-P., Cremer, J., et al. (2014). ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). European Heart Journal, 35(37), 2541–619. Oct 1.
Onuma, Y., Serruys, P. W., Perkins, L. E. L., Okamura, T., Gonzalo, N., Garcia-Garcia, H. M., et al. (2010). Intracoronary optical coherence tomography and histology at 1 month and 2, 3, and 4 years after implantation of everolimus-eluting bioresorbable vascular scaffolds in a porcine coronary artery model: an attempt to decipher the human optical coherence tomography images in the ABSORB trial. Circulation, 122(22), 2288–300.
Vert, M. (2009). Bioabsorbable polymers in medicine: an overview. EuroIntervention, 15(5), F9–F14.
Serruys, P. W., Ormiston, J. A., Onuma, Y., Regar, E., Gonzalo, N., Garcia-Garcia, H. M., et al. (2009). A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. The Lancet, 373(9667), 897–910 Elsevier Ltd.
Ribichini, F., Pighi, M., Faggian, G., Vassanelli, C. Bioresorbable vascular scaffolds in cardiac allograft vasculopathy: a new therapeutic option. AJM, 126(11):e11–4.
Lipinski, M. J., Escarcega, R. O., Lhermusier, T., & Waksman, R. (2014). The effects of novel, bioresorbable scaffolds on coronary vascular pathophysiology. Journal of Cardiovascular Translational Research, 7(4), 413–25.
Capranzano, P., Testa, L., Tamburino, C., Capodanno, D., Biondi-Zoccai, G., Longo, G., et al. (2014). Technical features of Absorb(TM) BVS implantation in the IT-DISAPPEARS registry. Giornale Italiano di Cardiologia (Rome), 15(9), 475–81.
Prati, F., Regar, E., Mintz, G. S., Arbustini, E., Di Mario, C., Jang, I. K., et al. (2010). Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. European Heart Journal, 31(4), 401–15.
Tearney, G. J., Regar, E., Akasaka, T., Adriaenssens, T., Barlis, P., Bezerra, H. G., et al. (2012). Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies. JAC, 59(12), 1058–72. Elsevier Inc.
Tuzcu, E. M., Kapadia, S. R., Tutar, E., Ziada, K. M., Hobbs, R. E., McCarthy, P. M., et al. (2001). High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation, 103(22), 2705–10.
Stone, G. W., Maehara, A., Lansky, A. J., de Bruyne, B., Cristea, E., Mintz, G. S., et al. (2011). A prospective natural-history study of coronary atherosclerosis. The New England Journal of Medicine, 364(3), 226–35.
Mehran, R., Aymong, E. D., Nikolsky, E., Lasic, Z., Iakovou, I., Fahy, M., et al. (2004). A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. Journal of the American Chemical Society, 44(7), 1393–9.
Weis, M., & von Scheidt, W. (2000). Coronary artery disease in the transplanted heart. Annual Review of Medicine, 51(1), 81–100.
Lund, L. H., Edwards, L. B., Kucheryavaya, A. Y., Benden, C., Christie, J. D., Dipchand, A. I., et al. (2014). The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report—2014; focus theme: retransplantation. HEALUN, 33(10), 996–1008. Elsevier.
Simpson, L., Lee, E. K., Hott, B. J., Vega, D. J., & Book, W. M. (2005). Long-term results of angioplasty vs stenting in cardiac transplant recipients with allograft vasculopathy. The Journal of Heart and Lung Transplantation, 24(9), 1211–7.
Schmauss, D., & Weis, M. (2008). Cardiac allograft vasculopathy: recent developments. Circulation [Internet], 117(16), 2131–41. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=18427143&retmode=ref&cmd=prlinks.
Benza, R. L., Zoghbi, G. J., Tallaj, J., Brown, R., Kirklin, J. K., Hubbard, M., et al. (2004). Palliation of allograft vasculopathy with transluminal angioplasty: a decade of experience. Journal of the American Chemical Society, 43(11), 1973–81.
Bader, F. M., Kfoury, A. G., Gilbert, E. M., Barry, W. H., Humayun, N., Hagan, M. E., et al. (2006). Percutaneous coronary interventions with stents in cardiac transplant recipients. The Journal of Heart and Lung Transplantation, 25(3), 298–301. Elsevier.
Berry, C., van’t Veer, M., Witt, N., Kala, P., Bocek, O., Pyxaras, S. A., et al. (2013). VERIFY (VERification of Instantaneous Wave-Free Ratio and Fractional Flow Reserve for the Assessment of Coronary Artery Stenosis Severity in EverydaY Practice): a multicenter study in consecutive patients. Journal of the American College of Cardiology, 61(13), 1421–7.
Gomez-Lara, J., Brugaletta, S., Farooq, V., Onuma, Y., Diletti, R., Windecker, S., et al. (2011). Head-to-head comparison of the neointimal response between metallic and bioresorbable everolimus-eluting scaffolds using optical coherence tomography. JACC. Cardiovascular Interventions, 4(12), 1271–80.
Ormiston, J. A., Serruys, P. W., Regar, E., Dudek, D., Thuesen, L., Webster, M. W., et al. (2008). A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet, 371(9616), 899–907.
Ormiston, J. A., Serruys, P. W., Onuma, Y., van Geuns, R. J., de Bruyne, B., Dudek, D., et al. (2012). First serial assessment at 6 months and 2 years of the second generation of absorb everolimus-eluting bioresorbable vascular scaffold: a multi-imaging modality study. Circulation: Cardiovascular Interventions, 5(5), 620–32. Lippincott Williams & Wilkins.
Brugaletta, S., Radu, M. D., Garcia-Garcia, H. M., Heo, J. H., Farooq, V., Girasis, C., et al. (2012). Circumferential evaluation of the neointima by optical coherence tomography after ABSORB bioresorbable vascular scaffold implantation: can the scaffold cap the plaque? Atherosclerosis, 221(1), 106–12. Elsevier.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Ethical Approval
All procedures performed in studies involving human participants are in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Associate Editor Emanuele Barbato oversaw the review of this article
Trial Registration
ClinicalTrials.gov registration number: NCT02377648 (24th February 2015)
URL: https://www.clinicaltrials.gov/ct2/show/NCT02377648
Rights and permissions
About this article
Cite this article
Pighi, M., Tomai, F., Petrolini, A. et al. Everolimus-Eluting Bioresorbable Vascular Scaffold System in the Treatment of Cardiac Allograft Vasculopathy: the CART (Cardiac Allograft Reparative Therapy) Prospective Multicenter Pilot Study. J. of Cardiovasc. Trans. Res. 9, 40–48 (2016). https://doi.org/10.1007/s12265-015-9665-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-015-9665-x